当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal Substitution-Induced Reducing Capacity of Magnetite Coupled with Aqueous Fe(II)
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-05-15 , DOI: 10.1021/acsearthspacechem.0c00089 Ying Li 1, 2, 3 , Gaoling Wei 4, 5 , Xiaoliang Liang 1, 3, 6 , Caihua Zhang 1 , Jianxi Zhu 1, 3, 6 , Yuji Arai 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-05-15 , DOI: 10.1021/acsearthspacechem.0c00089 Ying Li 1, 2, 3 , Gaoling Wei 4, 5 , Xiaoliang Liang 1, 3, 6 , Caihua Zhang 1 , Jianxi Zhu 1, 3, 6 , Yuji Arai 2
Affiliation
|
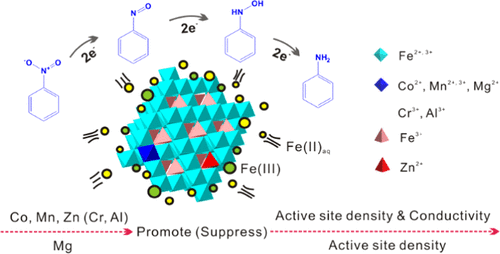
Aqueous Fe(II) (Fe(II)aq) effectively magnifies the reducibility of magnetite toward environmental substances. In natural magnetite, isomorphous substitution by foreign metals is ubiquitous, and Zn2+ and Co2+ have been reported to positively improve the reducing capacity of magnetite coupled with Fe(II)aq. Though most metal ions significantly alter the surface properties of magnetite, their effects on the reactivity of magnetite coupled with Fe(II)aq have rarely been systematically compared, resulting in the ambiguity of constraint mechanism and controlling factors. Herein, magnetites (Fe3–xMxO4, M = Co2+, Mn2+, Zn2+, Mg2+, Cr3+, and Al 3+) with similar substitution level (x ≈ 0.5) were synthesized, characterized, and tested for the reduction of nitrobenzene (NB) in the presence of Fe(II)aq. Both the reduction kinetics and the extent of electron transfer illustrated the positive effect of divalent metals but the negative effect of trivalent ones. Such distinct effects were further correlated to the physiochemical properties and microstructure of magnetite by the Pearson analysis. The active-site density and electrical conductivity of magnetite were critical factors determining the reduction performance of the coupled system. Specifically, Co, Mn, Zn, and Mg increased the active-site density and accordingly the adsorption capacity of Fe(II)aq. Moreover, the octahedral Mn and Co with thermodynamically favorable redox pairs, i.e., Co2+/Co3+ and Mn2+/Mn3+, accelerated electron exchange, giving rise to the increase of electrical conductivity. The tetrahedral Zn2+ induced the oxidation of octahedral Fe2+ to Fe3+, which also promoted the electron transfer. These results shed light on the role of natural magnetite and its impact on the fate of nitroaromatic compounds in anoxic environments.
中文翻译:

磁铁矿与Fe(II)水溶液耦合的金属取代诱导还原能力
Fe(II)(Fe(II)aq)水溶液有效地放大了磁铁矿对环境物质的还原性。在天然磁铁矿中,外来金属的同构取代是普遍存在的,据报道Zn 2+和Co 2+可以积极改善磁铁矿与Fe(II)水溶液的还原能力。尽管大多数金属离子会显着改变磁铁矿的表面性能,但很少系统地比较它们对与Fe(II)aq耦合的磁铁矿反应性的影响,从而导致约束机制和控制因素不明确。在此,磁铁矿(Fe 3– x M x O 4,M = Co 2+,锰2+,锌2+,镁2+,铬3+和Al 3+)具有类似取代水平(X ≈0.5)的合成,其特征在于,并测试其硝基苯的存在下还原(NB)铁(II)水溶液。还原动力学和电子转移程度都说明了二价金属的正作用,但说明了三价金属的负作用。通过皮尔森分析,这种不同的作用还与磁铁矿的理化性质和微观结构相关。磁铁矿的活性部位密度和电导率是决定耦合系统还原性能的关键因素。具体而言,Co,Mn,Zn和Mg会增加活性位点密度,从而增加Fe(II)aq的吸附能力。而且,具有热力学上有利的氧化还原对的八面体Mn和Co,即Co 2+ / Co 3+和Mn 2+ / Mn 3+促进电子交换,从而增加电导率。四面体Zn 2+诱导八面体Fe 2+氧化为Fe 3+,这也促进了电子转移。这些结果揭示了天然磁铁矿的作用及其对缺氧环境中硝基芳族化合物命运的影响。
更新日期:2020-06-18
中文翻译:

磁铁矿与Fe(II)水溶液耦合的金属取代诱导还原能力
Fe(II)(Fe(II)aq)水溶液有效地放大了磁铁矿对环境物质的还原性。在天然磁铁矿中,外来金属的同构取代是普遍存在的,据报道Zn 2+和Co 2+可以积极改善磁铁矿与Fe(II)水溶液的还原能力。尽管大多数金属离子会显着改变磁铁矿的表面性能,但很少系统地比较它们对与Fe(II)aq耦合的磁铁矿反应性的影响,从而导致约束机制和控制因素不明确。在此,磁铁矿(Fe 3– x M x O 4,M = Co 2+,锰2+,锌2+,镁2+,铬3+和Al 3+)具有类似取代水平(X ≈0.5)的合成,其特征在于,并测试其硝基苯的存在下还原(NB)铁(II)水溶液。还原动力学和电子转移程度都说明了二价金属的正作用,但说明了三价金属的负作用。通过皮尔森分析,这种不同的作用还与磁铁矿的理化性质和微观结构相关。磁铁矿的活性部位密度和电导率是决定耦合系统还原性能的关键因素。具体而言,Co,Mn,Zn和Mg会增加活性位点密度,从而增加Fe(II)aq的吸附能力。而且,具有热力学上有利的氧化还原对的八面体Mn和Co,即Co 2+ / Co 3+和Mn 2+ / Mn 3+促进电子交换,从而增加电导率。四面体Zn 2+诱导八面体Fe 2+氧化为Fe 3+,这也促进了电子转移。这些结果揭示了天然磁铁矿的作用及其对缺氧环境中硝基芳族化合物命运的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号