当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrophilic amination reactions with 1H‐indazole‐3‐carboxylates: Synthesis of amino acid frameworks and 3‐amino‐2‐oxindoles
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-05-14 , DOI: 10.1002/jhet.4015 Isao Mizota 1 , Mayuko Mori 1 , Makoto Shimizu 1, 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-05-14 , DOI: 10.1002/jhet.4015 Isao Mizota 1 , Mayuko Mori 1 , Makoto Shimizu 1, 2
Affiliation
|
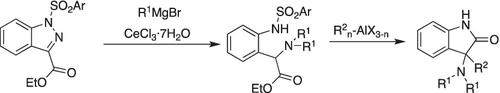
Reaction of 1‐ sulfonylindazole‐3‐carboxylates with various Grignard reagents effects the N ‐N bond cleavage of the hydrazone moiety with the first nucleophile and the subsequent N ‐alkylation gives N ,N ‐dialkylation products in good yields. A new strategy for the synthesis of α‐(2‐arylsulfonamide)phenylglycine, a precursor to tissue factor/factor VIIa inhibitors is also described. Moreover, the synthesis of quaternary 3‐aminooxindoles is developed, utilizing this N ,N ‐dialkylation reaction, followed by intramolecular cyclization/nucleophilic addition reaction.
中文翻译:

与1H-吲唑-3-羧酸盐的亲电胺化反应:氨基酸骨架和3-氨基-2-氧吲哚的合成
的1反应- sulfonylindazole -3-羧酸与各种格氏试剂效果Ñ - Ñ与所述第一亲核试剂腙部分的键裂解和随后的Ñ烷基化给出Ñ,Ñ -dialkylation制品以良好的收率。还描述了一种合成α-(2-芳基磺酰胺)苯基甘氨酸的新策略,α-(2-芳基磺酰胺)苯基甘氨酸是组织因子/因子VIIa抑制剂的前体。此外,利用该N,N-二烷基化反应,然后进行分子内环化/亲核加成反应,开发了季3-氨基羟吲哚的合成方法。
更新日期:2020-07-15
中文翻译:

与1H-吲唑-3-羧酸盐的亲电胺化反应:氨基酸骨架和3-氨基-2-氧吲哚的合成
的1反应- sulfonylindazole -3-羧酸与各种格氏试剂效果Ñ - Ñ与所述第一亲核试剂腙部分的键裂解和随后的Ñ烷基化给出Ñ,Ñ -dialkylation制品以良好的收率。还描述了一种合成α-(2-芳基磺酰胺)苯基甘氨酸的新策略,α-(2-芳基磺酰胺)苯基甘氨酸是组织因子/因子VIIa抑制剂的前体。此外,利用该N,N-二烷基化反应,然后进行分子内环化/亲核加成反应,开发了季3-氨基羟吲哚的合成方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号