Cell Host & Microbe ( IF 20.6 ) Pub Date : 2020-05-15 , DOI: 10.1016/j.chom.2020.04.013 Bidong D Nguyen 1 , Miguelangel Cuenca V 1 , Johannes Hartl 1 , Ersin Gül 1 , Rebekka Bauer 1 , Susanne Meile 1 , Joel Rüthi 1 , Céline Margot 1 , Laura Heeb 1 , Franziska Besser 1 , Pau Pérez Escriva 2 , Céline Fetz 1 , Markus Furter 1 , Leanid Laganenka 1 , Philipp Keller 1 , Lea Fuchs 1 , Matthias Christen 2 , Steffen Porwollik 3 , Michael McClelland 3 , Julia A Vorholt 1 , Uwe Sauer 2 , Shinichi Sunagawa 1 , Beat Christen 2 , Wolf-Dietrich Hardt 1
|
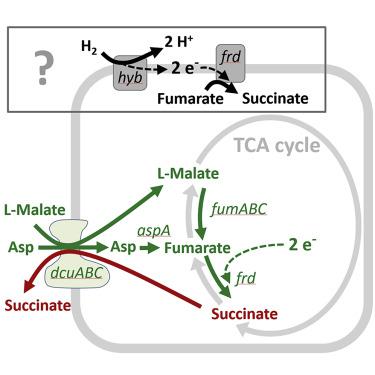
Initial enteropathogen growth in the microbiota-colonized gut is poorly understood. Salmonella Typhimurium is metabolically adaptable and can harvest energy by anaerobic respiration using microbiota-derived hydrogen (H2) as an electron donor and fumarate as an electron acceptor. As fumarate is scarce in the gut, the source of this electron acceptor is unclear. Here, transposon sequencing analysis along the colonization trajectory of S. Typhimurium implicates the C4-dicarboxylate antiporter DcuABC in early murine gut colonization. In competitive colonization assays, DcuABC and enzymes that convert the C4-dicarboxylates aspartate and malate into fumarate (AspA, FumABC), are required for fumarate/H2-dependent initial growth. Thus, S. Typhimurium obtains fumarate by DcuABC-mediated import and conversion of L-malate and L-aspartate. Fumarate reduction yields succinate, which is exported by DcuABC in exchange for L-aspartate and L-malate. This cycle allows S. Typhimurium to harvest energy by H2/fumarate respiration in the microbiota-colonized gut. This strategy may also be relevant for commensal E. coli diminishing the S. Typhimurium infection.
中文翻译:

DcuABC 输入天冬氨酸和苹果酸可驱动 H2/富马酸呼吸,促进沙门氏菌在小鼠肠腔内的初始定植。
肠道病原体在微生物群定植的肠道中的初始生长尚不清楚。鼠伤寒沙门氏菌具有代谢适应性,可以使用微生物群衍生的氢 (H 2 ) 作为电子供体和富马酸盐作为电子受体,通过无氧呼吸获取能量。由于富马酸盐在肠道中稀缺,因此这种电子受体的来源尚不清楚。在这里,沿着S的定植轨迹进行转座子测序分析。鼠伤寒菌表明 C4-二羧酸逆向转运蛋白 DcuABC 参与早期小鼠肠道定植。在竞争性定植测定中,DcuABC 和将 C4-二羧酸天冬氨酸和苹果酸转化为富马酸的酶(AspA、FumABC)是富马酸/H 2依赖性初始生长所必需的。因此,S . 鼠伤寒菌通过 DcuABC 介导的 L-苹果酸和 L-天冬氨酸的输入和转化获得富马酸。富马酸还原产生琥珀酸,由 DcuABC 出口以换取 L-天冬氨酸和 L-苹果酸。这个循环允许S . 鼠伤寒菌通过微生物群定植的肠道中的H 2 /富马酸盐呼吸来获取能量。该策略也可能与共生大肠杆菌减少S相关。鼠伤寒感染。











































 京公网安备 11010802027423号
京公网安备 11010802027423号