当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quinacrine Based Gold Hybrid Nanoparticles Caused Apoptosis through Modulating Replication Fork in Oral Cancer Stem Cells.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-14 , DOI: 10.1021/acs.molpharmaceut.0c00197 Krushna Chandra Hembram 1 , Somya Ranjan Dash 1 , Biswajit Das 1 , Chinmayee Sethy 1 , Subhajit Chatterjee 1 , Birendra Kumar Bindhani 2 , Chanakya Nath Kundu 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-14 , DOI: 10.1021/acs.molpharmaceut.0c00197 Krushna Chandra Hembram 1 , Somya Ranjan Dash 1 , Biswajit Das 1 , Chinmayee Sethy 1 , Subhajit Chatterjee 1 , Birendra Kumar Bindhani 2 , Chanakya Nath Kundu 1
Affiliation
|
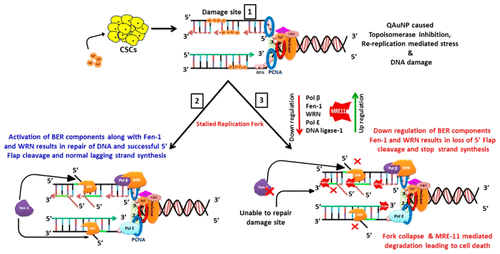
The presence of cancer stem cells (CSCs) in the tumor microenvironment is responsible for the development of chemoresistance and recurrence of cancer. Our previous investigation revealed the anticancer mechanism of quinacrine-based silver and gold hybrid nanoparticles (QAgNP and QAuNP) in oral cancer cells, but to avoid cancer recurrence, it is important to study the effect of these nanoparticles (NPs) on CSCs. Here, we developed an in vitro CSCs model using SCC-9 oral cancer cells and validated via FACS analysis. Then, 40–60% of cells were found to be CD44+/CD133+ and CD24–. QAuNP showed excellent anti-CSC growth potential against SCC-9-cancer stem like cells (IC50 = 0.4 μg/mL) with the down-regulation of representative CSC markers. Prolonged exposure of QAuNP induced the S-phase arrest and caused re-replication shown by the extended G2/M population and apoptosis to SCC-9-CSC like cells. Up-regulation of BAX, PARP cleavage, and simultaneous down-regulation of Bcl-xL in prolonged treatment to CSCs suggested that the majority of the cells have undergone apoptosis. QAuNP treatment also caused a loss in DNA repair in CSCs. Mostly, the base excision repair (BER) components (Fen-1, DNA ligase-1, Pol-β, RPA, etc.) were significantly down-regulated after QAuNP treatment, which suggested its action against DNA repair machinery. The replication fork maintenance-related proteins, RAD 51 and BRCA-2, were also deregulated. Very surprisingly, depletion of WRN (an interacting partner for Pre-RC and Fen-1) and a significant increase in expression of fork-degrading nuclease MRE-11 in 96 h treated NPs were observed. Results suggest QAuNP treatment caused excessive DNA damage and re-replication mediated replication stress (RS) and stalling of the replication fork. Inhibition of BER components hinders the flap clearance activity of Fen-1, and it further caused RS and stopped DNA synthesis. Overall, QAuNP treatment led to irreparable replication fork movement, and the stalled replication fork might have degraded by MRE-11, which ultimately results in apoptosis and the death of the CSCs.
中文翻译:

奎纳克林为基础的金杂化纳米颗粒通过调节口腔癌干细胞中的复制叉引起凋亡。
肿瘤微环境中癌症干细胞(CSC)的存在是导致化学抗药性和癌症复发的原因。我们以前的研究揭示了奎纳克林为基础的银和金杂化纳米颗粒(QAgNP和QAuNP)在口腔癌细胞中的抗癌机制,但为避免癌症复发,研究这些纳米颗粒(NPs)对CSC的作用很重要。在这里,我们使用SCC-9口腔癌细胞建立了体外CSCs模型,并通过FACS分析进行了验证。然后,发现40-60%的细胞是CD44 + / CD133 +和CD24-。QAuNP对SCC-9癌干样细胞显示出极好的抗CSC生长潜力(IC 50= 0.4μg/ mL),且具有代表性的CSC标志物下调。长时间暴露于QAuNP会诱导S期阻滞,并导致G2 / M种群扩大和SCC-9-CSC样细胞凋亡导致的复制。在延长对CSC的治疗中,BAX的上调,PARP的切割以及Bcl-xL的同时下调提示大多数细胞已经凋亡。QAuNP处理还导致CSC中DNA修复的损失。通常,在QAuNP处理后,碱基切除修复(BER)组件(Fen-1,DNA连接酶-1,Pol-β,RPA等)被显着下调,表明其对DNA修复机制的作用。复制叉维持相关蛋白RAD 51和BRCA-2也被解除了调控。非常令人惊讶 观察到WRN(Pre-RC和Fen-1的相互作用伴侣)的耗竭,以及在96小时处理的NP中叉降解核酸酶MRE-11的表达显着增加。结果表明,QAuNP处理导致过度的DNA损伤,以及由复制介导的复制压力(RS)和复制叉的停滞。BER成分的抑制会阻碍Fen-1的皮瓣清除活性,并进一步引起RS并停止DNA合成。总体而言,QAuNP治疗导致复制叉运动无法挽回,停滞的复制叉可能已被MRE-11降解,最终导致细胞凋亡和CSC死亡。结果表明,QAuNP处理导致过度的DNA损伤,以及由复制介导的复制压力(RS)和复制叉的停滞。BER成分的抑制会阻碍Fen-1的皮瓣清除活性,并进一步引起RS并停止DNA合成。总体而言,QAuNP治疗导致无法修复的复制叉运动,并且停滞的复制叉可能已被MRE-11降解,最终导致细胞凋亡和CSC死亡。结果表明,QAuNP处理导致过度的DNA损伤,以及由复制介导的复制压力(RS)和复制叉的停滞。BER成分的抑制会阻碍Fen-1的皮瓣清除活性,并进一步引起RS并停止DNA合成。总体而言,QAuNP治疗导致复制叉运动无法挽回,停滞的复制叉可能已被MRE-11降解,最终导致细胞凋亡和CSC死亡。
更新日期:2020-07-06
中文翻译:

奎纳克林为基础的金杂化纳米颗粒通过调节口腔癌干细胞中的复制叉引起凋亡。
肿瘤微环境中癌症干细胞(CSC)的存在是导致化学抗药性和癌症复发的原因。我们以前的研究揭示了奎纳克林为基础的银和金杂化纳米颗粒(QAgNP和QAuNP)在口腔癌细胞中的抗癌机制,但为避免癌症复发,研究这些纳米颗粒(NPs)对CSC的作用很重要。在这里,我们使用SCC-9口腔癌细胞建立了体外CSCs模型,并通过FACS分析进行了验证。然后,发现40-60%的细胞是CD44 + / CD133 +和CD24-。QAuNP对SCC-9癌干样细胞显示出极好的抗CSC生长潜力(IC 50= 0.4μg/ mL),且具有代表性的CSC标志物下调。长时间暴露于QAuNP会诱导S期阻滞,并导致G2 / M种群扩大和SCC-9-CSC样细胞凋亡导致的复制。在延长对CSC的治疗中,BAX的上调,PARP的切割以及Bcl-xL的同时下调提示大多数细胞已经凋亡。QAuNP处理还导致CSC中DNA修复的损失。通常,在QAuNP处理后,碱基切除修复(BER)组件(Fen-1,DNA连接酶-1,Pol-β,RPA等)被显着下调,表明其对DNA修复机制的作用。复制叉维持相关蛋白RAD 51和BRCA-2也被解除了调控。非常令人惊讶 观察到WRN(Pre-RC和Fen-1的相互作用伴侣)的耗竭,以及在96小时处理的NP中叉降解核酸酶MRE-11的表达显着增加。结果表明,QAuNP处理导致过度的DNA损伤,以及由复制介导的复制压力(RS)和复制叉的停滞。BER成分的抑制会阻碍Fen-1的皮瓣清除活性,并进一步引起RS并停止DNA合成。总体而言,QAuNP治疗导致复制叉运动无法挽回,停滞的复制叉可能已被MRE-11降解,最终导致细胞凋亡和CSC死亡。结果表明,QAuNP处理导致过度的DNA损伤,以及由复制介导的复制压力(RS)和复制叉的停滞。BER成分的抑制会阻碍Fen-1的皮瓣清除活性,并进一步引起RS并停止DNA合成。总体而言,QAuNP治疗导致无法修复的复制叉运动,并且停滞的复制叉可能已被MRE-11降解,最终导致细胞凋亡和CSC死亡。结果表明,QAuNP处理导致过度的DNA损伤,以及由复制介导的复制压力(RS)和复制叉的停滞。BER成分的抑制会阻碍Fen-1的皮瓣清除活性,并进一步引起RS并停止DNA合成。总体而言,QAuNP治疗导致复制叉运动无法挽回,停滞的复制叉可能已被MRE-11降解,最终导致细胞凋亡和CSC死亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号