当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exotic Electrophiles in Chlorinated and Chloraminated Water: When Conventional Kinetic Models and Reaction Pathways Fall Short
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2020-05-14 , DOI: 10.1021/acs.estlett.0c00259 Michael R. Rose 1 , Stephanie S. Lau 2 , Carsten Prasse 3 , John D. Sivey 4, 5
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2020-05-14 , DOI: 10.1021/acs.estlett.0c00259 Michael R. Rose 1 , Stephanie S. Lau 2 , Carsten Prasse 3 , John D. Sivey 4, 5
Affiliation
|
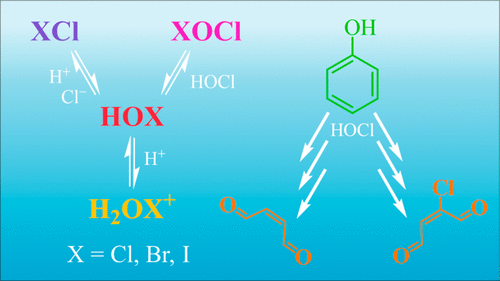
Halogenation and oxidation of organic matter in chlorinated and chloraminated water are typically attributed to the most abundant electrophiles present. This interpretation sometimes fails to explain laboratory observations, including halogenation kinetics and product distributions. Exotic electrophiles, species commonly overlooked in the environmental literature, can help to resolve these discrepancies. Herein, we review evidence demonstrating the significance of lesser-studied electrophilic chlorinating (Cl2 and Cl2O), brominating (BrCl, BrOCl, and Br2O), and iodinating (H2OI+ and ICl) agents in chlor(am)inated water. The evidence includes reaction rate dependencies on [Cl–], [H+], and [HOCl] that cannot be attributed to the reactivity of hypohalous acids or hypohalites alone. For example, enhancement of chlorination and bromination rates by Cl– implicates Cl2 and BrCl, respectively, as active halogenating agents. Herein, we discuss a new method for quantifying the sensitivity of halogenation to rate enhancement by Cl–. We also discuss complexities that Cl– can impart on iodination kinetics. In addition, we highlight recent insights into radical-mediated reaction pathways and unexpected organic electrophiles in chlorinated water. Finally, we discuss practical implications, identify research needs, and offer recommendations to improve the design of future halogenation experiments. Overall, this review aims to spur new research into underappreciated electrophiles in chlor(am)inated water.
中文翻译:

氯化和氯化水中的外来亲电子试剂:传统动力学模型和反应途径不足时
氯化水中和氯化水中有机物的卤化和氧化通常归因于存在的最丰富的亲电子试剂。这种解释有时无法解释实验室的观察结果,包括卤化动力学和产物分布。外来亲电试剂是环境文献中通常忽略的物种,可以帮助解决这些差异。在本文中,我们回顾了证据,这些证据表明氯(am2)中亲电性氯化(Cl 2和Cl 2 O),溴化(BrCl,BrOCl和Br 2 O)和碘化(H 2 OI +和ICl)试剂的重要性)水。证据包括反应速率对[Cl - ],[H+ ]和[HOCl]不能仅归因于次卤酸或次卤酸盐的反应性。例如,增强的经Cl氯化和溴化率-牵连氯2分别和氯化溴,作为活性的卤化剂。在这里,我们讨论了一种量化Cl –对卤代对速率增强的敏感性的新方法。我们还讨论了Cl –可以赋予碘化动力学。此外,我们重点介绍了有关自由基介导的反应途径和氯化水中意外的有机亲电子试剂的最新见解。最后,我们讨论了实际意义,确定研究需求并提供建议以改进未来卤化实验的设计。总的来说,这篇综述的目的是刺激对含氯(am)的水中未被充分认识的亲电子试剂的新研究。
更新日期:2020-05-14
中文翻译:

氯化和氯化水中的外来亲电子试剂:传统动力学模型和反应途径不足时
氯化水中和氯化水中有机物的卤化和氧化通常归因于存在的最丰富的亲电子试剂。这种解释有时无法解释实验室的观察结果,包括卤化动力学和产物分布。外来亲电试剂是环境文献中通常忽略的物种,可以帮助解决这些差异。在本文中,我们回顾了证据,这些证据表明氯(am2)中亲电性氯化(Cl 2和Cl 2 O),溴化(BrCl,BrOCl和Br 2 O)和碘化(H 2 OI +和ICl)试剂的重要性)水。证据包括反应速率对[Cl - ],[H+ ]和[HOCl]不能仅归因于次卤酸或次卤酸盐的反应性。例如,增强的经Cl氯化和溴化率-牵连氯2分别和氯化溴,作为活性的卤化剂。在这里,我们讨论了一种量化Cl –对卤代对速率增强的敏感性的新方法。我们还讨论了Cl –可以赋予碘化动力学。此外,我们重点介绍了有关自由基介导的反应途径和氯化水中意外的有机亲电子试剂的最新见解。最后,我们讨论了实际意义,确定研究需求并提供建议以改进未来卤化实验的设计。总的来说,这篇综述的目的是刺激对含氯(am)的水中未被充分认识的亲电子试剂的新研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号