当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Validation and Mechanism of a Low-Cost Graphite Carbon Electrode for Electrochemical Brine Valorization
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-05-13 , DOI: 10.1021/acssuschemeng.0c01485 Linchao Mu 1 , Yichong Wang 1 , William A. Tarpeh 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-05-13 , DOI: 10.1021/acssuschemeng.0c01485 Linchao Mu 1 , Yichong Wang 1 , William A. Tarpeh 1
Affiliation
|
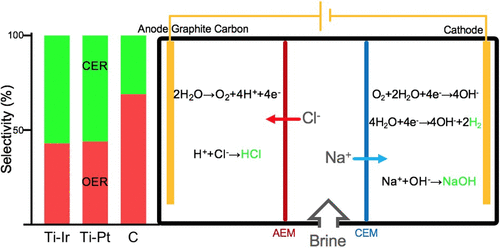
Desalination adoption outpaces the development of effective strategies for managing the concentrated brine streams it produces. Electrochemical water-salt splitting to generate useful chemicals is an ideal method to valorize wastewater from desalination and incentivize its collection, which can expand the adoption of desalination to inland regions. We designed a three-chamber electrochemical cell that splits brine to generate an acid (HCl) and a base (NaOH). The three chambers were separated by an anion exchange membrane (AEM) and a cation exchange membrane (CEM), and the reactor operated robustly in various realistic brine concentrations (13,000–35,000 ppm NaCl). Buffer species, including carbonate, borate, and phosphate, reduced proton leakage through the AEM and did not introduce other side reactions. By testing several anodes for stability and selectivity toward oxygen evolution, we found that graphite carbon exhibited a 25% higher acid generation than mixed-metal oxides (Ti–Ir and Ti–Pt) and a 69% selectivity toward oxygen evolution because of a lower chloride adsorption on its surface (44% less chloride oxidized). This study advances material development, reactor design, and mechanistic understanding for electrochemical salt-water splitting to produce commodity chemicals from desalination brine.
中文翻译:

一种低成本用于电化学盐水平衡的石墨碳电极的验证及机理
采用海水淡化技术的速度超过了管理其产生的浓盐水流的有效策略的发展速度。电化学水盐分离法产生有用的化学物质是一种理想的方法,可以使脱盐废水中的水保持平衡并促进其收集,从而可以将脱盐技术推广到内陆地区。我们设计了一个三腔电化学池,该池将盐水分流以产生酸(HCl)和碱(NaOH)。三个腔室被阴离子交换膜(AEM)和阳离子交换膜(CEM)隔开,反应器在各种实际盐水浓度(13,000–35,000 ppm NaCl)中都能稳定运行。缓冲剂,包括碳酸盐,硼酸盐和磷酸盐,可减少质子通过AEM的泄漏,并且不会引起其他副反应。通过测试几个阳极的稳定性和对氧气析出的选择性,我们发现石墨碳比混合金属氧化物(Ti–Ir和Ti–Pt)具有更高的酸生成率25%,并且由于较低的析出率,其对氧气释放的选择性高达69%。氯化物在其表面上的吸附(氧化的氯化物少44%)。这项研究促进了材料开发,反应器设计以及对电化学盐水分离以从淡化盐水中生产商品化学品的机理的理解。
更新日期:2020-05-13
中文翻译:

一种低成本用于电化学盐水平衡的石墨碳电极的验证及机理
采用海水淡化技术的速度超过了管理其产生的浓盐水流的有效策略的发展速度。电化学水盐分离法产生有用的化学物质是一种理想的方法,可以使脱盐废水中的水保持平衡并促进其收集,从而可以将脱盐技术推广到内陆地区。我们设计了一个三腔电化学池,该池将盐水分流以产生酸(HCl)和碱(NaOH)。三个腔室被阴离子交换膜(AEM)和阳离子交换膜(CEM)隔开,反应器在各种实际盐水浓度(13,000–35,000 ppm NaCl)中都能稳定运行。缓冲剂,包括碳酸盐,硼酸盐和磷酸盐,可减少质子通过AEM的泄漏,并且不会引起其他副反应。通过测试几个阳极的稳定性和对氧气析出的选择性,我们发现石墨碳比混合金属氧化物(Ti–Ir和Ti–Pt)具有更高的酸生成率25%,并且由于较低的析出率,其对氧气释放的选择性高达69%。氯化物在其表面上的吸附(氧化的氯化物少44%)。这项研究促进了材料开发,反应器设计以及对电化学盐水分离以从淡化盐水中生产商品化学品的机理的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号