当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer.
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-05-14 , DOI: 10.1002/jcp.29784 Han Guan 1 , Rui Peng 1 , Fang Fang 2 , Likai Mao 3 , Zhijun Chen 1 , Shuai Yang 1 , Changyuan Dai 1 , Hongliang Wu 1 , Chengyong Wang 1 , Ninghan Feng 4 , Bin Xu 5 , Ming Chen 5
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-05-14 , DOI: 10.1002/jcp.29784 Han Guan 1 , Rui Peng 1 , Fang Fang 2 , Likai Mao 3 , Zhijun Chen 1 , Shuai Yang 1 , Changyuan Dai 1 , Hongliang Wu 1 , Chengyong Wang 1 , Ninghan Feng 4 , Bin Xu 5 , Ming Chen 5
Affiliation
|
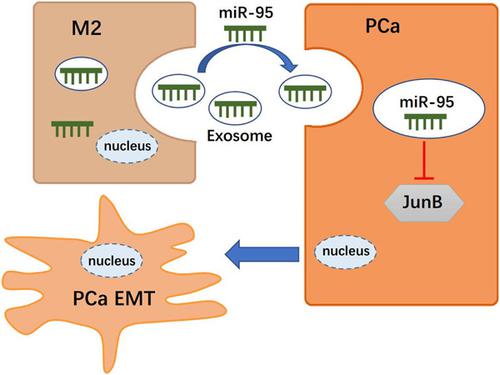
Tumor‐associated macrophages (TAMs) are vital constituents in mediating cell‐to‐cell communication within the tumor microenvironment. However, the molecular mechanisms underlying the interplay between TAMs and tumor cells that guide cell fate are largely undetermined. Extracellular vesicles, also known as exosomes, which are derived from TAMs, are the components exerting regulatory effects. Thus, understanding the underlying mechanism of “onco‐vesicles” is of crucial importance for prostate cancer (PCa) therapy. In this study, we analyzed micro RNA sequences in exosomes released by THP‐1 and M2 macrophages and found a significant increase in miR‐95 levels in TAM‐derived exosomes, demonstrating the direct uptake of miR‐95 by recipient PCa cells. In vitro and in vivo loss‐of‐function assays suggested that miR‐95 could function as a tumor promoter by directly binding to its downstream target gene, JunB, to promote PCa cell proliferation, invasion, and epithelial–mesenchymal transition. The clinical data analyses further revealed that higher miR‐95 expression results in worse clinicopathological features. Collectively, our results demonstrated that TAM‐mediated PCa progression is partially attributed to the aberrant expression of miR‐95 in TAM‐derived exosomes, and the miR‐95/JunB axis provides the groundwork for research on TAMs to further develop more‐personalized therapeutic approaches for patients with PCa.
中文翻译:

肿瘤相关的巨噬细胞通过外来体介导的miR-95转移促进前列腺癌的进展。
肿瘤相关巨噬细胞(TAM)是介导肿瘤微环境内细胞间通讯的重要组成部分。但是,在TAM和引导细胞命运的肿瘤细胞之间相互作用的潜在分子机制尚不清楚。源自TAM的细胞外囊泡(也称为外来体)是发挥调节作用的成分。因此,了解“囊泡”的潜在机制对于前列腺癌(PCa)治疗至关重要。在这项研究中,我们分析了THP-1和M2巨噬细胞释放的外泌体中的微小RNA序列,发现TAM衍生的外泌体中miR-95的水平显着增加,表明受体PCa细胞直接摄取miR-95。体外和体内功能丧失试验表明,miR-95可通过直接与其下游靶基因JunB结合来促进PCa细胞增殖,侵袭和上皮-间质转化,从而充当肿瘤启动子。临床数据分析进一步表明,较高的miR-95表达导致较差的临床病理特征。总的来说,我们的结果表明,TAM介导的PCa进程部分归因于TAM衍生的外泌体中miR-95的异常表达,而miR-95 / JunB轴为TAM的研究奠定基础,以进一步开发更具个性化的治疗方法PCa患者的治疗方法。临床数据分析进一步表明,较高的miR-95表达导致较差的临床病理特征。总的来说,我们的结果表明,TAM介导的PCa进程部分归因于TAM衍生的外泌体中miR-95的异常表达,而miR-95 / JunB轴为TAM的研究奠定基础,以进一步开发更具个性化的治疗方法PCa患者的治疗方法。临床数据分析进一步表明,较高的miR-95表达导致较差的临床病理特征。总的来说,我们的结果表明,TAM介导的PCa进程部分归因于TAM衍生的外泌体中miR-95的异常表达,而miR-95 / JunB轴为TAM的研究奠定基础,以进一步开发更具个性化的治疗方法PCa患者的治疗方法。
更新日期:2020-05-14
中文翻译:

肿瘤相关的巨噬细胞通过外来体介导的miR-95转移促进前列腺癌的进展。
肿瘤相关巨噬细胞(TAM)是介导肿瘤微环境内细胞间通讯的重要组成部分。但是,在TAM和引导细胞命运的肿瘤细胞之间相互作用的潜在分子机制尚不清楚。源自TAM的细胞外囊泡(也称为外来体)是发挥调节作用的成分。因此,了解“囊泡”的潜在机制对于前列腺癌(PCa)治疗至关重要。在这项研究中,我们分析了THP-1和M2巨噬细胞释放的外泌体中的微小RNA序列,发现TAM衍生的外泌体中miR-95的水平显着增加,表明受体PCa细胞直接摄取miR-95。体外和体内功能丧失试验表明,miR-95可通过直接与其下游靶基因JunB结合来促进PCa细胞增殖,侵袭和上皮-间质转化,从而充当肿瘤启动子。临床数据分析进一步表明,较高的miR-95表达导致较差的临床病理特征。总的来说,我们的结果表明,TAM介导的PCa进程部分归因于TAM衍生的外泌体中miR-95的异常表达,而miR-95 / JunB轴为TAM的研究奠定基础,以进一步开发更具个性化的治疗方法PCa患者的治疗方法。临床数据分析进一步表明,较高的miR-95表达导致较差的临床病理特征。总的来说,我们的结果表明,TAM介导的PCa进程部分归因于TAM衍生的外泌体中miR-95的异常表达,而miR-95 / JunB轴为TAM的研究奠定基础,以进一步开发更具个性化的治疗方法PCa患者的治疗方法。临床数据分析进一步表明,较高的miR-95表达导致较差的临床病理特征。总的来说,我们的结果表明,TAM介导的PCa进程部分归因于TAM衍生的外泌体中miR-95的异常表达,而miR-95 / JunB轴为TAM的研究奠定基础,以进一步开发更具个性化的治疗方法PCa患者的治疗方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号