Materials Today Energy ( IF 9.0 ) Pub Date : 2020-05-14 , DOI: 10.1016/j.mtener.2020.100437 H.A. Younus , M. Vandichel , N. Ahmad , E. Ahlberg , M. Busch , F. Verpoort
|
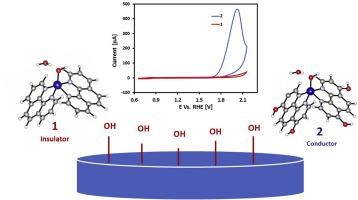
Water oxidation is traditionally performed over IrO2 and RuO2 owing to their high stability at low pH compared to molecular O2 evolution catalysts. The low stability of molecular complexes in acids limits their industrial exploitation as anodes in water-splitting devices, where high current densities and proton conductivity are required. Herein, an existing Co(1,10-phenanthroline)2 complex film is engineered to improve its pH-stability via extra OH substituents on the ligand, i.e. 1,10-phenanthroline-4,7-diol. This novel Co(1,10-phenanthroline-4,7-diol)2 complex film is active for water oxidation at low overpotentials and stable at low pH. Since the calculated water oxidation overpotentials of both complexes are similar, the difference in water oxidation activity is attributed to a smaller charge transfer resistance, which originates from a different anchoring style to the electrode via the OH groups of the ligand. This result is supported by electrochemical impedance measurements. The high pH-stability of the Co(1,10-phenanthroline-4,7-diol)2 film is computationally rationalized by a high crystal formation energy observed in DFT calculations. In summary, an acid-stable and active cobalt-based metal-organic film is reported that competes well with most reported earth-abundant catalysts for water oxidation under similar conditions.
中文翻译:

高度稳定的金属有机共膜工程设计,可在酸性介质中有效地电催化水氧化
由于与分子O 2析出催化剂相比在低pH下具有较高的稳定性,因此传统上会在IrO 2和RuO 2上进行水氧化。酸中分子配合物的低稳定性限制了它们在水分解装置中作为阳极的工业应用,在水分解装置中,需要高电流密度和质子传导性。本文中,现有的Co(1,10-菲咯啉)2复合膜经过工程设计,可通过配体上的额外OH取代基(即1,10-菲咯啉-4,7-二醇)提高其pH稳定性。这个新颖的Co(1,10-菲咯啉-4,7-二醇)2复合膜在低电势下对水氧化有活性,在低pH下稳定。由于两种配合物的水氧化超电势相似,因此水氧化活性的差异归因于较小的电荷转移阻力,这是由于通过配体的OH基团对电极的锚固方式不同而引起的。该结果得到电化学阻抗测量的支持。Co(1,10-菲咯啉-4,7-二醇)2膜的高pH稳定性通过DFT计算中观察到的高晶体形成能在计算上得到合理化。总而言之,据报道,在相似的条件下,酸稳定的钴基活性金属有机薄膜可与大多数报道的富含地球的催化剂竞争水氧化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号