Chem ( IF 19.1 ) Pub Date : 2020-05-14 , DOI: 10.1016/j.chempr.2020.04.014 Yang Yu , Ji-Min Yang , Julius Rebek
|
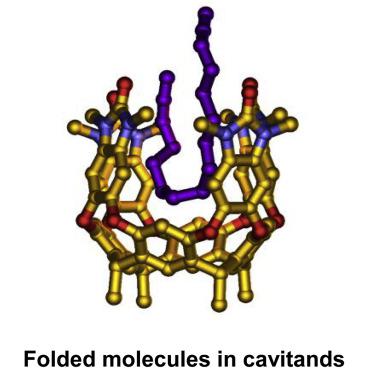
Template effects are at the origin of supramolecular chemistry, but the behavior of folded molecules is a relatively new undertaking. Water-soluble cavitand hosts bind hydrocarbons through hydrophobic effects and force long-chain guests into folded conformations. This brings their ends closer together, and sites that were remote in solution become neighbors in the confined space and affect each other’s reactivity. Amphiphilic guests fold in the cavitand to bury hydrophobic surfaces and expose the hydrophilic surfaces to the bulk solution. This arrangement leads to product distributions in monofunctionalization reactions that are significantly altered from the statistically determined outcomes in solution. The cavitand also acts as a template for macrocyclic processes involving direct reaction of the guests’ ends. We propose applying the effects of folding in cavitands to truly remote functionalization reactions and provide access to molecules that cannot be made by conventional means.
中文翻译:

密闭空间中的分子:Cavitands中的反应性和可能性
模板效应是超分子化学的起源,但是折叠分子的行为是一个相对较新的事业。水溶性空洞主体通过疏水作用结合碳氢化合物,并迫使长链客体变成折叠构象。这使他们的两端更加接近,解决方案中距离较远的站点成为受限空间中的邻居,并影响彼此的反应性。两性客体在空洞中折叠以掩埋疏水表面,并使亲水表面暴露于本体溶液。这种安排导致单官能化反应中的产物分布与溶液中统计确定的结果有很大不同。该cavitand还充当涉及客体末端直接反应的大环过程的模板。











































 京公网安备 11010802027423号
京公网安备 11010802027423号