Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-05-14 , DOI: 10.1016/j.chembiol.2020.04.004 Edward P Harvey 1 , Zachary J Hauseman 1 , Daniel T Cohen 1 , T Justin Rettenmaier 2 , Susan Lee 1 , Annissa J Huhn 1 , Thomas E Wales 3 , Hyuk-Soo Seo 4 , James Luccarelli 1 , Catherine E Newman 1 , Rachel M Guerra 1 , Gregory H Bird 1 , Sirano Dhe-Paganon 4 , John R Engen 3 , James A Wells 2 , Loren D Walensky 1
|
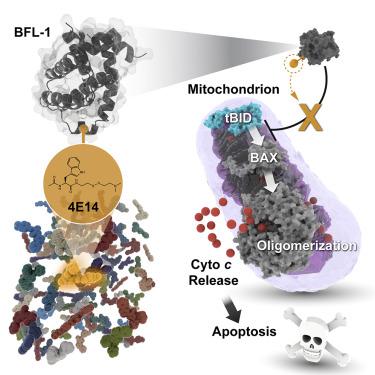
The BCL-2 family is composed of anti- and pro-apoptotic members that respectively protect or disrupt mitochondrial integrity. Anti-apoptotic overexpression can promote oncogenesis by trapping the BCL-2 homology 3 (BH3) “killer domains” of pro-apoptotic proteins in a surface groove, blocking apoptosis. Groove inhibitors, such as the relatively large BCL-2 drug venetoclax (868 Da), have emerged as cancer therapies. BFL-1 remains an undrugged oncogenic protein and can cause venetoclax resistance. Having identified a unique C55 residue in the BFL-1 groove, we performed a disulfide tethering screen to determine if C55 reactivity could enable smaller molecules to block BFL-1's BH3-binding functionality. We found that a disulfide-bearing N-acetyltryptophan analog (304 Da adduct) effectively targeted BFL-1 C55 and reversed BFL-1-mediated suppression of mitochondrial apoptosis. Structural analyses implicated the conserved leucine-binding pocket of BFL-1 as the interaction site, resulting in conformational remodeling. Thus, therapeutic targeting of BFL-1 may be achievable through the design of small, cysteine-reactive drugs.
中文翻译:

通过二硫键绑定鉴定抗凋亡BFL-1的共价分子抑制剂。
BCL-2家族由分别保护或破坏线粒体完整性的抗凋亡成员和促凋亡成员组成。抗凋亡过度表达可通过在表面凹槽中捕获促凋亡蛋白的BCL-2同源性3(BH3)“杀手域”来促进肿瘤发生,从而阻止凋亡。沟槽抑制剂,例如相对较大的BCL-2药物Venetoclax(868 Da),已作为癌症疗法出现。BFL-1仍然是一种不发毒的致癌蛋白,并可能导致耐胶膜粘连。在确定BFL-1凹槽中独特的C55残基后,我们进行了二硫键束缚筛选,以确定C55反应性是否可以使较小的分子阻断BFL-1的BH3结合功能。我们发现带有二硫键的N-乙酰基色氨酸类似物(304 Da加合物)有效靶向BFL-1 C55,并逆转了BFL-1介导的线粒体凋亡抑制。结构分析暗示BFL-1的保守的亮氨酸结合口袋作为相互作用位点,导致构象重塑。因此,可以通过设计小的半胱氨酸反应性药物来实现BFL-1的治疗靶向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号