当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Fmoc-solid-phase peptide synthesis of an extracellular loop of CFTR for antibody selection by the phage display technology.
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2020-05-12 , DOI: 10.1002/psc.3253 Vera F C Ferreira 1 , João D G Correia 1, 2 , Carlos M Farinha 3 , Filipa Mendes 1, 2
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2020-05-12 , DOI: 10.1002/psc.3253 Vera F C Ferreira 1 , João D G Correia 1, 2 , Carlos M Farinha 3 , Filipa Mendes 1, 2
Affiliation
|
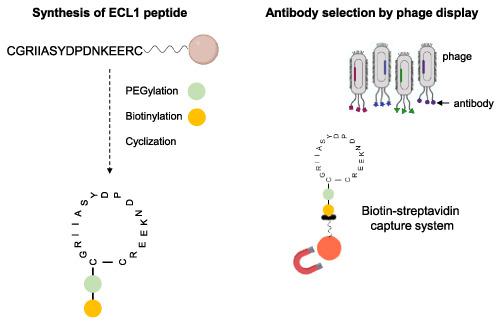
Cystic fibrosis (CF), a life‐shortening genetic disease, is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene that codes for the CFTR protein, the major chloride channel expressed at the apical membrane of epithelial cells. The development of an imaging probe capable of non‐invasively detect CFTR at the cell surface could be of great advantage for the management of CF. With that purpose, we synthesized the first extracellular loop of CFTR protein (ECL1) through fluorenylmethyloxycarbonyl (Fmoc)‐based microwave‐assisted solid‐phase peptide synthesis (SPPS), according to a reported methodology. However, aspartimide formation, a well‐characterized side reaction in Fmoc‐SPPS, prompted us to adopt a different side‐chain protection strategy for aspartic acid residues present in ECL1 sequence. The peptide was subsequently modified via PEGylation and biotinylation, and cyclized through disulfide bridge formation, mimicking the native loop conformation in CFTR protein. Herein, we report improvements in the synthesis of the first extracellular loop of CFTR, including peptide modifications that can be used to improve antigen presentation in phage display for selection of novel antibodies against plasma membrane CFTR.
中文翻译:

CFTR胞外环的改良Fmoc-固相肽合成,可通过噬菌体展示技术进行抗体选择。
囊性纤维化(CF)是缩短寿命的遗传病,是由CFTR跨膜电导调节剂(CFTR)基因的突变引起的,该基因编码CFTR蛋白,CFTR蛋白是在上皮细胞顶膜表达的主要氯离子通道。能够无创检测细胞表面CFTR的成像探针的开发对于CF的管理可能具有很大的优势。为此,根据报道的方法,我们通过基于芴基甲氧基羰基(Fmoc)的微波辅助固相肽合成(SPPS)合成了CFTR蛋白(ECL1)的第一个胞外环。然而,Fmoc-SPPS中天冬酰胺的形成是一个特征明确的副反应,促使我们对ECL1序列中存在的天冬氨酸残基采用不同的侧链保护策略。随后通过PEG化和生物素化修饰该肽,并通过二硫键形成环化,从而模拟CFTR蛋白中的天然环构象。在本文中,我们报道了CFTR的第一个细胞外环的合成方面的改进,包括可用于改善噬菌体展示中抗原呈递的肽修饰,以选择针对质膜CFTR的新型抗体。
更新日期:2020-05-12
中文翻译:

CFTR胞外环的改良Fmoc-固相肽合成,可通过噬菌体展示技术进行抗体选择。
囊性纤维化(CF)是缩短寿命的遗传病,是由CFTR跨膜电导调节剂(CFTR)基因的突变引起的,该基因编码CFTR蛋白,CFTR蛋白是在上皮细胞顶膜表达的主要氯离子通道。能够无创检测细胞表面CFTR的成像探针的开发对于CF的管理可能具有很大的优势。为此,根据报道的方法,我们通过基于芴基甲氧基羰基(Fmoc)的微波辅助固相肽合成(SPPS)合成了CFTR蛋白(ECL1)的第一个胞外环。然而,Fmoc-SPPS中天冬酰胺的形成是一个特征明确的副反应,促使我们对ECL1序列中存在的天冬氨酸残基采用不同的侧链保护策略。随后通过PEG化和生物素化修饰该肽,并通过二硫键形成环化,从而模拟CFTR蛋白中的天然环构象。在本文中,我们报道了CFTR的第一个细胞外环的合成方面的改进,包括可用于改善噬菌体展示中抗原呈递的肽修饰,以选择针对质膜CFTR的新型抗体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号