Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-05-13 , DOI: 10.1016/j.bbamem.2020.183354 Matthias Schuster 1 , Mattia Deluigi 2 , Milica Pantić 1 , Santiago Vacca 2 , Christian Baumann 1 , Daniel J Scott 3 , Andreas Plückthun 2 , Oliver Zerbe 1
|
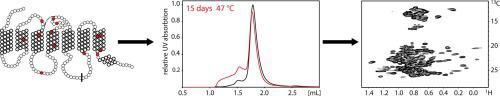
Sample preparation for NMR studies of G protein-coupled receptors faces special requirements: Proteins need to be stable for prolonged measurements at elevated temperatures, they should ideally be uniformly labeled with the stable isotopes 13C, 15N, and all carbon-bound protons should be replaced by deuterons. In addition, certain NMR experiments require protonated methyl groups in the presence of a perdeuterated background. All these requirements are most easily satisfied when using Escherichia coli as the expression host. Here we describe a workflow, starting from a temperature-stabilized mutant of the α1B-adrenergic receptor, obtained using the CHESS methodology, into an even more stable species, in which flexible parts from termini were removed and the intracellular loop 3 (ICL3) was stabilized against proteolytic cleavage. The yield after purification corresponds to 1–2 mg/L of D2O culture. The final purification step is ligand-affinity chromatography to ensure that only well-folded ligand-binding protein is isolated. Proper selection of detergent has a remarkable influence on the quality of NMR spectra. All optimization steps of sequence and detergent are monitored on a small scale by monitoring the melting temperature and long-term thermal stability to allow for screening of many conditions. The stabilized mutant of the α1B-adrenergic receptor was additionally incorporated in nanodiscs, but displayed slightly inferior spectra compared to a sample in detergent micelles. Finally, both [15N,1H]- as well as [13C,1H]-HSQC spectra are shown highlighting the high quality of the final NMR sample. Importantly, the quality of [13C,1H]-HSQC spectra indicates that the so prepared receptor could be used for studying side-chain dynamics.
中文翻译:

优化用于溶液NMR研究的α1B-肾上腺素能受体。
用于G蛋白偶联受体的NMR研究的样品制备面临特殊要求:蛋白质需要在高温下长时间测量时保持稳定,理想情况下,应使用稳定的同位素13 C,15 N统一标记它们,并且所有与碳键合的质子应被氘核取代。另外,某些NMR实验需要在氘化背景下存在质子化的甲基。当使用大肠杆菌作为表达宿主时,最容易满足所有这些要求。在这里,我们描述一个工作流程,从开始温度稳定的突变体的α的1B用CHESS方法获得的β-肾上腺素能受体成为一种更加稳定的物种,其中去除了末端的柔性部分,并稳定了细胞内环3(ICL3)的抗蛋白水解作用。纯化后的产量相当于1-2 mg / L的D 2 O培养物。最终的纯化步骤是配体亲和色谱法,以确保仅分离折叠良好的配体结合蛋白。正确选择洗涤剂对NMR谱图质量有显着影响。通过监控熔融温度和长期热稳定性,可以对多种条件进行筛选,从而对序列和洗涤剂的所有优化步骤进行小规模监控。稳定化的突变体的α的1B-肾上腺素能受体还被掺入了纳米圆盘中,但与去污剂胶束中的样品相比,其光谱显示稍差。最后,[ 15 N,1 H]-以及[ 13 C,1 H] -HSQC光谱均得到了展示,突出了最终NMR样品的高质量。重要的是,[ 13 C,1 H] -HSQC光谱的质量表明,如此制备的受体可用于研究侧链动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号