当前位置:
X-MOL 学术
›
J. Mol. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The nature of multiple boron-nitrogen bonds studied using electron localization function (ELF), electron density (AIM), and natural bond orbital (NBO) methods.
Journal of Molecular Modeling ( IF 2.1 ) Pub Date : 2020-05-13 , DOI: 10.1007/s00894-020-04374-9 Grzegorz Mierzwa 1 , Agnieszka J Gordon 1 , Slawomir Berski 1
Journal of Molecular Modeling ( IF 2.1 ) Pub Date : 2020-05-13 , DOI: 10.1007/s00894-020-04374-9 Grzegorz Mierzwa 1 , Agnieszka J Gordon 1 , Slawomir Berski 1
Affiliation
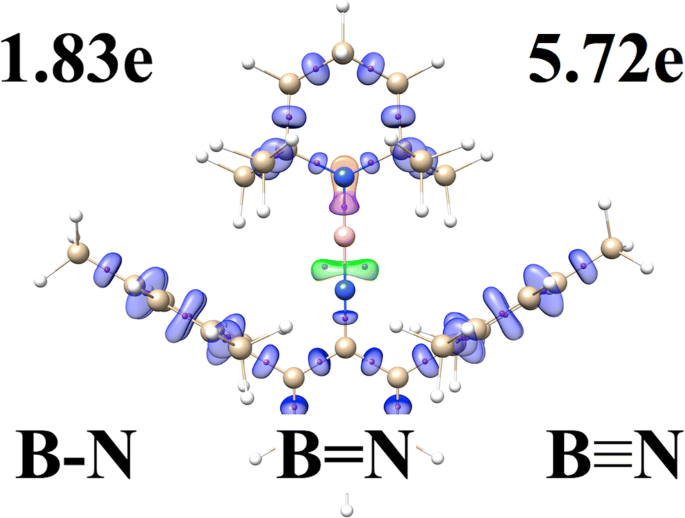
Local nature of the boron-nitrogen (BN) bonding with different formal multiplicities (B≡N, B=N, B-N) have been investigated for 25 experimentally established organoboron molecules in both real and the Hilbert space, using topological analysis of electron localization function (ELF), electron density (AIM), and natural bond orbital (NBO) method. Each BN bond has been represented (ELF) by the bonding disynaptic attractor V(B,N), with the basin electron population between 5.72e and 1.83e, confirming possible existence of all the three bond types. A covalent character of bonding can be associated with the dative mechanism due to the V(B,N) bonding basin formed mainly (91–96%) by the N electron density. Similarly, the NBO method shows 2-center natural orbitals, consisting largely of the hybrids from the N atom. The AIM analysis yields the features typical for shared (H(3,−1)(r) < 0) and closed-shell (∇2ρ(3,−1)(r) > 0) interactions. The delocalization indices, describing electron exchanges between B and N quantum atoms, are smaller than 1.5, even for formally very short triple B≡N bonds.
 .
.
中文翻译:

使用电子定位函数 (ELF)、电子密度 (AIM) 和自然键轨道 (NBO) 方法研究多重硼氮键的性质。
使用电子定位函数的拓扑分析,研究了真实空间和希尔伯特空间中 25 个实验建立的有机硼分子具有不同形式多重性(B=N、B=N、BN)的硼-氮(BN)键的局域性质(ELF)、电子密度(AIM)和自然键轨道(NBO)方法。每个 BN 键都由键合双突触吸引子 V(B,N) 表示 (ELF),盆地电子布居数在 5.72e 和 1.83e 之间,证实了所有三种键类型的可能存在。由于 V(B,N) 键合盆地主要 (91-96%) 由 N 电子密度形成,因此键合的共价特征可以与配位机制相关。类似地,NBO 方法显示了 2 中心自然轨道,主要由 N 原子的杂化物组成。 AIM 分析产生共享 (H (3,−1) ( r ) < 0) 和闭壳 (∇ 2 ρ (3,−1) ( r ) > 0) 相互作用的典型特征。描述 B 和 N 量子原子之间的电子交换的离域指数小于 1.5,即使对于形式上非常短的三 BeqN 键也是如此。 .
.
更新日期:2020-05-13
 .
.中文翻译:

使用电子定位函数 (ELF)、电子密度 (AIM) 和自然键轨道 (NBO) 方法研究多重硼氮键的性质。
使用电子定位函数的拓扑分析,研究了真实空间和希尔伯特空间中 25 个实验建立的有机硼分子具有不同形式多重性(B=N、B=N、BN)的硼-氮(BN)键的局域性质(ELF)、电子密度(AIM)和自然键轨道(NBO)方法。每个 BN 键都由键合双突触吸引子 V(B,N) 表示 (ELF),盆地电子布居数在 5.72e 和 1.83e 之间,证实了所有三种键类型的可能存在。由于 V(B,N) 键合盆地主要 (91-96%) 由 N 电子密度形成,因此键合的共价特征可以与配位机制相关。类似地,NBO 方法显示了 2 中心自然轨道,主要由 N 原子的杂化物组成。 AIM 分析产生共享 (H (3,−1) ( r ) < 0) 和闭壳 (∇ 2 ρ (3,−1) ( r ) > 0) 相互作用的典型特征。描述 B 和 N 量子原子之间的电子交换的离域指数小于 1.5,即使对于形式上非常短的三 BeqN 键也是如此。
 .
.










































 京公网安备 11010802027423号
京公网安备 11010802027423号