当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Does the degree of substitution on the cyclodextrin hosts impact their affinity towards guest binding?
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-05-13 , DOI: 10.1039/d0pp00103a Goutam Chakraborty 1 , Alok K Ray 1, 2 , Prabhat K Singh 2, 3 , Haridas Pal 2, 3
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-05-13 , DOI: 10.1039/d0pp00103a Goutam Chakraborty 1 , Alok K Ray 1, 2 , Prabhat K Singh 2, 3 , Haridas Pal 2, 3
Affiliation
|
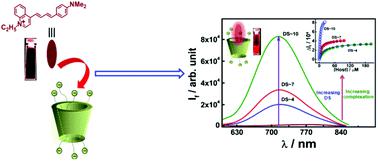
Although cyclodextrins have been extensively utilized in various branches of supramolecular chemistry due to their numerous attractive attributes, however, to achieve even advanced applications, they often need structural modification through substitutions of suitable functional groups at their rims. A systematic investigation on how the degree of substitution on the cyclodextrin rims affects the binding affinity for a given guest molecule has however rarely been reported, especially from the perspective of photophysical studies. Herein, we report the non-covalent interaction of a styryl based dye, LDS-798, with three different sulfobutylether beta cyclodextrin (SBEnβCD) derivatives bearing varying degrees of substitution (n), using ground state absorption, steady-state emission, excited-state lifetime and time-resolved fluorescence anisotropy measurements. The dye–host binding constant values indicate that the strength of the interaction between LDS-798 and SBEnβCD derivatives follows an increasing trend with an increasing number of tethered sulfobutylether substituents on the cyclodextrin rims, which is attributed to the gradual increase of the electrostatic interaction between the negatively charged sulfobutylether groups and the positively charged LDS-798. Excited state lifetime measurements and ionic strength dependent studies on the dye–SBEnβCD complexes further support the increased affinity between the dye and the host in the supramolecular complexes, with an increasing number of sulfobutylether substituents on the βCD rims. The obtained results suggest that the molecular recognition of LDS-798 with SBEnβCD derivatives can be tuned very effectively by varying the number of sulfobutylether substituents on the cyclodextrin rims. Considering that SBE7βCD is one of the FDA approved agents for drug formulations, the obtained results with other SBEnβCD hosts may be useful in designing selective drug delivery applications, drug formulations, and effective fluorescence on–off switches.
中文翻译:

环糊精宿主上的取代度是否会影响其对客体结合的亲和力?
尽管环糊精由于其众多吸引人的特性而已在超分子化学的各个分支中得到广泛利用,但是,要实现更先进的应用,它们通常需要通过在其边缘取代合适的官能团来进行结构修饰。然而,很少有关于环糊精边缘上的取代度如何影响给定客体分子的结合亲和力的系统研究的报道,特别是从光物理研究的角度。在本文中,我们报道了苯乙烯基染料的非共价相互作用,LDS-798,具有三个不同的磺丁基醚β-环糊精(SBE Ñ βCD)衍生物轴承不同程度的取代(Ñ),使用基态吸收,稳态发射,激发态寿命和时间分辨荧光各向异性测量。染料主机结合常数值指示LDS-798和SBE之间的相互作用的强度Ñ βCD衍生物如下与环糊精边缘越来越多的系留磺丁基醚取代基,这归因于静电的逐渐增加有增加的趋势带负电荷的磺丁基醚基与带正电荷的LDS-798之间的相互作用。染料–SBE n的激发态寿命测量和离子强度依赖性研究βCD络合物进一步支持超分子络合物中染料与主体之间亲和力的增加,并且βCD边缘上的磺基丁基醚取代基数量增加。将所得到的结果表明,LDS-798与SBE的分子识别Ñ βCD衍生物可通过改变取代基的磺丁基醚对环糊精轮辋的数量非常有效地调节。考虑到SBE 7 βCD是FDA的一个批准用于药物配方剂,将获得的与其它SBE结果Ñ βCD主机可以是在设计选择性药物递送应用,药物制剂和有效荧光通断开关是有用的。
更新日期:2020-07-15
中文翻译:

环糊精宿主上的取代度是否会影响其对客体结合的亲和力?
尽管环糊精由于其众多吸引人的特性而已在超分子化学的各个分支中得到广泛利用,但是,要实现更先进的应用,它们通常需要通过在其边缘取代合适的官能团来进行结构修饰。然而,很少有关于环糊精边缘上的取代度如何影响给定客体分子的结合亲和力的系统研究的报道,特别是从光物理研究的角度。在本文中,我们报道了苯乙烯基染料的非共价相互作用,LDS-798,具有三个不同的磺丁基醚β-环糊精(SBE Ñ βCD)衍生物轴承不同程度的取代(Ñ),使用基态吸收,稳态发射,激发态寿命和时间分辨荧光各向异性测量。染料主机结合常数值指示LDS-798和SBE之间的相互作用的强度Ñ βCD衍生物如下与环糊精边缘越来越多的系留磺丁基醚取代基,这归因于静电的逐渐增加有增加的趋势带负电荷的磺丁基醚基与带正电荷的LDS-798之间的相互作用。染料–SBE n的激发态寿命测量和离子强度依赖性研究βCD络合物进一步支持超分子络合物中染料与主体之间亲和力的增加,并且βCD边缘上的磺基丁基醚取代基数量增加。将所得到的结果表明,LDS-798与SBE的分子识别Ñ βCD衍生物可通过改变取代基的磺丁基醚对环糊精轮辋的数量非常有效地调节。考虑到SBE 7 βCD是FDA的一个批准用于药物配方剂,将获得的与其它SBE结果Ñ βCD主机可以是在设计选择性药物递送应用,药物制剂和有效荧光通断开关是有用的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号