当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A microfluidics-derived growth factor gradient in a scaffold regulates stem cell activities for tendon-to-bone interface healing.
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-05-13 , DOI: 10.1039/d0bm00229a Jingtong Lyu 1 , Long Chen , Jiqiang Zhang , Xia Kang , Yunjiao Wang , Wenjie Wu , Hong Tang , Jun Wu , Zhiyu He , Kanglai Tang
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-05-13 , DOI: 10.1039/d0bm00229a Jingtong Lyu 1 , Long Chen , Jiqiang Zhang , Xia Kang , Yunjiao Wang , Wenjie Wu , Hong Tang , Jun Wu , Zhiyu He , Kanglai Tang
Affiliation
|
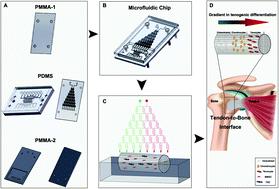
Treatment of tendon-to-bone interface injury has long been challenging in sports medicine. The major obstacle lies with the complicated three-layer structure of the tissue that consists of a bone region with osteocytes, a tendon region with tenocytes and a transitional region with chondrocytes. Conventional tissue engineering approaches using simply biomaterial scaffolds, stem cells and combinations of them had limited abilities to reconstruct the gradient structure with normal biomechanical properties. We herein aim to construct a three-layer structure with bone marrow-derived stem cells and tendon stem cells cultured in a decellularized tendon scaffold, through application of a gradient of biological cues in the longitudinal direction of the scaffold that guides the stem cells to differentiate and remodel the extracellular matrix in response to different medium concentrations in different regions. A microfluidic chip, on which a tree-like flow pattern was implemented, was adopted to create the concentration gradient in a dichotomous manner. We screened for an optimized seeding ratio between the two stem cell types before incubation of the scaffold in the medium concentration gradient and surgical implantation. Histology and immunohistochemistry assessments, both qualitatively and semi-quantitatively, showed that the microfluidic system provided desired guidance to the seeded stem cells that the healing at 8-week post-implantation presented a similar structure to that of a normal tendon-to-bone interface, which was outstanding compared to treatments without gradient guidance, stem cells or scaffolds where chaotic and fibrotic structures were obtained. This strategy offers a potentially translational tissue engineering approach for better outcomes in tendon-to-bone healing.
中文翻译:

支架中的微流体衍生生长因子梯度可调节干细胞的活动,以实现肌腱到骨骼的界面愈合。
长期以来,在运动医学中,腱-骨界面损伤的治疗一直具有挑战性。主要障碍在于组织的复杂三层结构,该结构由具有骨细胞的骨区域,具有肌腱细胞的肌腱区域和具有软骨细胞的过渡区域组成。仅使用生物材料支架,干细胞及其组合的常规组织工程方法重建具有正常生物力学特性的梯度结构的能力有限。我们的目标是构建具有骨髓来源的干细胞和在脱细胞的肌腱支架中培养的肌腱干细胞的三层结构,通过在支架的纵向方向上应用生物学线索的梯度来指导干细胞分化和重塑细胞外基质,以响应不同区域中的不同培养基浓度。采用在其上实现了树状流动模式的微流控芯片以二分方式创建浓度梯度。我们在培养基浓度梯度和手术植入中孵育支架之前,筛选了两种干细胞类型之间的最佳接种率。定性和半定量的组织学和免疫组化评估,结果表明,微流体系统为种子干细胞提供了理想的指导,即植入后8周的愈合表现出与正常肌腱到骨骼界面相似的结构,与没有梯度指导的治疗相比,这种方法非常出色。获得混乱和纤维化结构的细胞或支架。该策略提供了一种潜在的平移组织工程方法,可在腱到骨的愈合中获得更好的结果。
更新日期:2020-06-30
中文翻译:

支架中的微流体衍生生长因子梯度可调节干细胞的活动,以实现肌腱到骨骼的界面愈合。
长期以来,在运动医学中,腱-骨界面损伤的治疗一直具有挑战性。主要障碍在于组织的复杂三层结构,该结构由具有骨细胞的骨区域,具有肌腱细胞的肌腱区域和具有软骨细胞的过渡区域组成。仅使用生物材料支架,干细胞及其组合的常规组织工程方法重建具有正常生物力学特性的梯度结构的能力有限。我们的目标是构建具有骨髓来源的干细胞和在脱细胞的肌腱支架中培养的肌腱干细胞的三层结构,通过在支架的纵向方向上应用生物学线索的梯度来指导干细胞分化和重塑细胞外基质,以响应不同区域中的不同培养基浓度。采用在其上实现了树状流动模式的微流控芯片以二分方式创建浓度梯度。我们在培养基浓度梯度和手术植入中孵育支架之前,筛选了两种干细胞类型之间的最佳接种率。定性和半定量的组织学和免疫组化评估,结果表明,微流体系统为种子干细胞提供了理想的指导,即植入后8周的愈合表现出与正常肌腱到骨骼界面相似的结构,与没有梯度指导的治疗相比,这种方法非常出色。获得混乱和纤维化结构的细胞或支架。该策略提供了一种潜在的平移组织工程方法,可在腱到骨的愈合中获得更好的结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号