当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Factors Governing the Formation of Extended Structures in Group 10 and 12 Metal-Dithiolenes
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.cgd.0c00362 Oscar Castillo 1, 2 , Esther Delgado 3 , Elisa Hernández 3 , Maria Pérez 3 , Félix Zamora 3, 4
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.cgd.0c00362 Oscar Castillo 1, 2 , Esther Delgado 3 , Elisa Hernández 3 , Maria Pérez 3 , Félix Zamora 3, 4
Affiliation
|
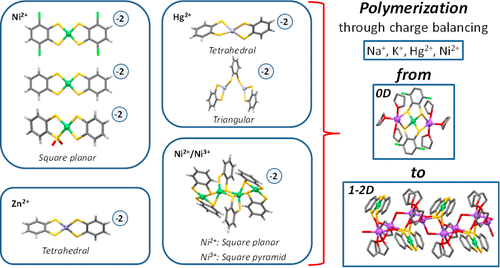
A series of metal-dithiolene compounds have been prepared by direct reactions between MCl2·6H2O (M = Ni, Zn) or HgCl2 with HSC6H2X2SH (X = Cl or H) in the presence of a base. The fine-tuning of the reaction conditions has allowed us to rationalize part of the structural factors governing the formation of coordination polymers versus discrete metal-dithiolene complexes. With the aim to gain knowledge of the formation of extended metal–organic structures, we have also studied the reaction between Na[Ni(SC6H2Cl2S)2] and HgCl2. These reactions lead to the formation of either discrete compounds or extended structures. The detailed structural analysis of 11 compounds has allowed us to uncover some relevant clues about the formation of coordination polymers based on dithiolenes. We have observed that extended structures are typically formed when: (i) the sulfur atoms apart from being involved in the chelation of the transition metal center also coordinate to the alkaline metal center; i.e., dithiolene acts as a bridging ligand; (ii) water molecules preferentially coordinate to alkaline centers mostly acting as bridging molecules; and (iii) bulkier alkaline cations are employed to allow a greater number of sulfur atoms coming from the metal-dithiolene entities to be incorporated in their coordination sphere. The results obtained ensure that a combination of at least two of these features gives rise to polymeric coordination entities.
中文翻译:

控制第10和第12组金属-二硫代烯烃扩展结构形成的结构因素
MCl 2 ·6H 2 O(M = Ni,Zn)或HgCl 2与HSC 6 H 2 X 2 SH(X = Cl或H)之间直接反应制得了一系列金属二硫代化合物。基础。反应条件的微调使我们能够合理地控制支配聚合物与离散金属-二硫代烯烃配合物形成的部分结构因素。为了了解扩展的金属有机结构的形成,我们还研究了Na [Ni(SC 6 H 2 Cl 2 S)2 ]与HgCl 2之间的反应。这些反应导致离散化合物或扩展结构的形成。对11种化合物进行详细的结构分析,使我们得以发现一些有关基于二硫代烯烃形成配位聚合物的线索。我们已经观察到,通常在以下情况下形成扩展结构:(i)除了与过渡金属中心的螯合有关之外,硫原子还与碱金属中心配位;即,二硫烯起桥连配体的作用;(ii)水分子优先与主要充当桥连分子的碱中心配位;(iii)使用更大的碱性阳离子,以使更多的来自金属-二硫代烯烃实体的硫原子结合到它们的配位域中。
更新日期:2020-07-01
中文翻译:

控制第10和第12组金属-二硫代烯烃扩展结构形成的结构因素
MCl 2 ·6H 2 O(M = Ni,Zn)或HgCl 2与HSC 6 H 2 X 2 SH(X = Cl或H)之间直接反应制得了一系列金属二硫代化合物。基础。反应条件的微调使我们能够合理地控制支配聚合物与离散金属-二硫代烯烃配合物形成的部分结构因素。为了了解扩展的金属有机结构的形成,我们还研究了Na [Ni(SC 6 H 2 Cl 2 S)2 ]与HgCl 2之间的反应。这些反应导致离散化合物或扩展结构的形成。对11种化合物进行详细的结构分析,使我们得以发现一些有关基于二硫代烯烃形成配位聚合物的线索。我们已经观察到,通常在以下情况下形成扩展结构:(i)除了与过渡金属中心的螯合有关之外,硫原子还与碱金属中心配位;即,二硫烯起桥连配体的作用;(ii)水分子优先与主要充当桥连分子的碱中心配位;(iii)使用更大的碱性阳离子,以使更多的来自金属-二硫代烯烃实体的硫原子结合到它们的配位域中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号