当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon-Carbon Bond Cleavage at Allylic Positions: Retro-allylation and Deallylation.
Chemical Reviews ( IF 51.4 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.chemrev.0c00157 Keisuke Nogi 1 , Hideki Yorimitsu 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.chemrev.0c00157 Keisuke Nogi 1 , Hideki Yorimitsu 1
Affiliation
|
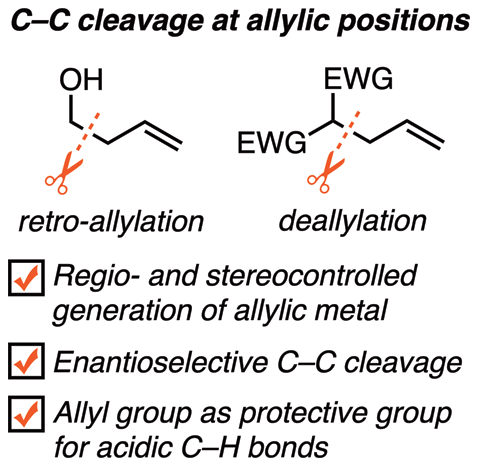
The development of C–C bond-cleaving transformations is an issue in modern organic chemistry that is as challenging as it is important. Among these transformations, the retro-allylation and deallylation of allylic compounds are uniquely intriguing methods for the cleavage of C–C σ bonds at the allylic position. Retro-allylation is regarded as a prospective method for the generation of highly valuable regio- and stereodefined allylic metal compounds. Because the C–C cleavage proceeds via a favorable six-membered chairlike transition state, the regio- and stereochemical information on the starting homoallylic alcohols can be transferred onto the products. Moreover, retro-allylation can also be achieved using enantioselective C–C cleavage powered by chiral catalysts for the synthesis of enantiomerically enriched compounds. As a result of these attractive features, retro-allylation has wide utility in regio-, stereo-, and enantioselective synthesis. Deallylation is C–C σ-bond cleavage involving the departure of an allylic fragment and the formation of a relatively stable carbanion or radical, and it proceeds via either oxidative addition to a low-valent metal or an addition/β-elimination cascade. The removal of the versatile allylic group might seem to be unproductive; however, this unique transformation offers the opportunity of using the allylic group as a protective group for acidic C–H bonds. This Review aims to exhibit the synthetic utility as well as the uniqueness of these two C–C σ-bond cleavage methods by presenting a wide range of transformations of allylic compounds with the aid of main group metals, transition-metal catalysts, and radical species.
中文翻译:

烯丙基位置处的碳-碳键裂解:逆烯丙基化和烯丙基化。
碳-碳键断裂转化的发展是现代有机化学中的一个问题,既具有挑战性,又具有重要意义。在这些转化中,烯丙基化合物的逆烯丙基化和脱烯丙基化是在烯丙基位置裂解C–Cσ键的独特方法。逆烯丙基化被认为是产生高度有价值的区域和立体定义的烯丙基金属化合物的预期方法。由于C–C裂解是通过有利的六元椅子状过渡态进行的,因此可以将起始均烯丙基醇的区域和立体化学信息转移到产品上。此外,还可以使用手性催化剂驱动的对映选择性C–C裂解来合成对映体富集化合物,实现逆烯丙基化。由于这些吸引人的特征,逆烯丙基化在区域,立体和对映选择性合成中具有广泛的用途。脱芳基化为C–Cσ键裂解,涉及到一个烯丙基片段的离去和相对稳定的碳负离子或自由基的形成,它通过氧化加成到低价金属或加成/β-消除级联反应进行。多才多艺的盟友集团的撤离似乎无济于事。然而,这种独特的转变提供了使用烯丙基作为酸性CH键的保护基的机会。这篇综述旨在通过主要金属族,过渡金属催化剂和自由基种类的广泛呈现,实现烯丙基化合物的转化,以展示这两种C–Cσ键裂解方法的合成效用以及其独特性。 。
更新日期:2020-05-12
中文翻译:

烯丙基位置处的碳-碳键裂解:逆烯丙基化和烯丙基化。
碳-碳键断裂转化的发展是现代有机化学中的一个问题,既具有挑战性,又具有重要意义。在这些转化中,烯丙基化合物的逆烯丙基化和脱烯丙基化是在烯丙基位置裂解C–Cσ键的独特方法。逆烯丙基化被认为是产生高度有价值的区域和立体定义的烯丙基金属化合物的预期方法。由于C–C裂解是通过有利的六元椅子状过渡态进行的,因此可以将起始均烯丙基醇的区域和立体化学信息转移到产品上。此外,还可以使用手性催化剂驱动的对映选择性C–C裂解来合成对映体富集化合物,实现逆烯丙基化。由于这些吸引人的特征,逆烯丙基化在区域,立体和对映选择性合成中具有广泛的用途。脱芳基化为C–Cσ键裂解,涉及到一个烯丙基片段的离去和相对稳定的碳负离子或自由基的形成,它通过氧化加成到低价金属或加成/β-消除级联反应进行。多才多艺的盟友集团的撤离似乎无济于事。然而,这种独特的转变提供了使用烯丙基作为酸性CH键的保护基的机会。这篇综述旨在通过主要金属族,过渡金属催化剂和自由基种类的广泛呈现,实现烯丙基化合物的转化,以展示这两种C–Cσ键裂解方法的合成效用以及其独特性。 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号