当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Optimization of the Lysine-Isopeptide Bond Forming Sortase Enzyme from Corynebacterium diphtheriae.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.bioconjchem.0c00163 Christopher K. Sue , Scott A. McConnell , Ken Ellis-Guardiola , John M. Muroski , Rachel A. McAllister , Justin Yu , Ana I. Alvarez , Chungyu Chang , Rachel R. Ogorzalek Loo , Joseph A. Loo , Hung Ton-That , Robert T. Clubb
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.bioconjchem.0c00163 Christopher K. Sue , Scott A. McConnell , Ken Ellis-Guardiola , John M. Muroski , Rachel A. McAllister , Justin Yu , Ana I. Alvarez , Chungyu Chang , Rachel R. Ogorzalek Loo , Joseph A. Loo , Hung Ton-That , Robert T. Clubb
|
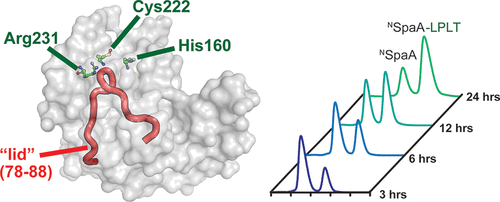
Site-specifically modified protein bioconjugates have important applications in biology, chemistry, and medicine. Functionalizing specific protein side chains with enzymes using mild reaction conditions is of significant interest, but remains challenging. Recently, the lysine–isopeptide bond forming activity of the sortase enzyme that builds surface pili in Corynebacterium diphtheriae (CdSrtA) has been reconstituted in vitro. A mutationally activated form of CdSrtA was shown to be a promising bioconjugating enzyme that can attach Leu-Pro-Leu-Thr-Gly peptide fluorophores to a specific lysine residue within the N-terminal domain of the SpaA protein (NSpaA), enabling the labeling of target proteins that are fused to NSpaA. Here we present a detailed analysis of the CdSrtA catalyzed protein labeling reaction. We show that the first step in catalysis is rate limiting, which is the formation of the CdSrtA-peptide thioacyl intermediate that subsequently reacts with a lysine ε-amine in NSpaA. This intermediate is surprisingly stable, limiting spurious proteolysis of the peptide substrate. We report the discovery of a new enzyme variant (CdSrtAΔ) that has significantly improved transpeptidation activity, because it completely lacks an inhibitory polypeptide appendage (“lid”) that normally masks the active site. We show that the presence of the lid primarily impairs formation of the thioacyl intermediate and not the recognition of the NSpaA substrate. Quantitative measurements reveal that CdSrtAΔ generates its cross-linked product with a catalytic turnover number of 1.4 ± 0.004 h–1 and that it has apparent KM values of 0.16 ± 0.04 and 1.6 ± 0.3 mM for its NSpaA and peptide substrates, respectively. CdSrtAΔ is 7-fold more active than previously studied variants, labeling >90% of NSpaA with peptide within 6 h. The results of this study further improve the utility of CdSrtA as a protein labeling tool and provide insight into the enzyme catalyzed reaction that underpins protein labeling and pilus biogenesis.
中文翻译:

白喉棒杆菌赖氨酸异肽键形成分选酶的动力学和优化。
位点特异性修饰的蛋白质生物缀合物在生物学、化学和医学中具有重要的应用。在温和的反应条件下用酶功能化特定的蛋白质侧链具有重要意义,但仍然具有挑战性。最近,在白喉棒状杆菌(Cd SrtA)中构建表面菌毛的分选酶的赖氨酸-异肽键形成活性已在体外重建。Cd SrtA的突变激活形式被证明是一种有前途的生物共轭酶,可以将 Leu-Pro-Leu-Thr-Gly 肽荧光团附着到 SpaA 蛋白 ( N SpaA) N 端结构域内的特定赖氨酸残基上,从而使与N SpaA融合的目标蛋白的标记。在这里,我们对Cd SrtA 催化的蛋白质标记反应进行了详细分析。我们表明催化的第一步是限速,即形成Cd SrtA-肽硫酰基中间体,随后与N SpaA中的赖氨酸 ε-胺反应。该中间体非常稳定,限制了肽底物的虚假蛋白水解。我们报告发现了一种新的酶变体(Cd SrtA Δ),它显着提高了转肽活性,因为它完全缺乏通常掩盖活性位点的抑制性多肽附加物(“盖子”)。我们表明盖子的存在主要损害硫酰基中间体的形成,而不是N SpaA 底物的识别。定量测量表明,Cd SrtA Δ生成的交联产物的催化周转数为 1.4 ± 0.004 h –1,其N SpaA 和肽底物的表观K M值为 0.16 ± 0.04 和 1.6 ± 0.3 mM ,分别。Cd SrtA Δ的活性比之前研究的变体高 7 倍,可在 6 小时内用肽标记 > 90% 的N SpaA。这项研究的结果进一步提高了Cd SrtA 作为蛋白质标记工具的实用性,并提供了对支撑蛋白质标记和菌毛生物发生的酶催化反应的深入了解。
更新日期:2020-05-12
中文翻译:

白喉棒杆菌赖氨酸异肽键形成分选酶的动力学和优化。
位点特异性修饰的蛋白质生物缀合物在生物学、化学和医学中具有重要的应用。在温和的反应条件下用酶功能化特定的蛋白质侧链具有重要意义,但仍然具有挑战性。最近,在白喉棒状杆菌(Cd SrtA)中构建表面菌毛的分选酶的赖氨酸-异肽键形成活性已在体外重建。Cd SrtA的突变激活形式被证明是一种有前途的生物共轭酶,可以将 Leu-Pro-Leu-Thr-Gly 肽荧光团附着到 SpaA 蛋白 ( N SpaA) N 端结构域内的特定赖氨酸残基上,从而使与N SpaA融合的目标蛋白的标记。在这里,我们对Cd SrtA 催化的蛋白质标记反应进行了详细分析。我们表明催化的第一步是限速,即形成Cd SrtA-肽硫酰基中间体,随后与N SpaA中的赖氨酸 ε-胺反应。该中间体非常稳定,限制了肽底物的虚假蛋白水解。我们报告发现了一种新的酶变体(Cd SrtA Δ),它显着提高了转肽活性,因为它完全缺乏通常掩盖活性位点的抑制性多肽附加物(“盖子”)。我们表明盖子的存在主要损害硫酰基中间体的形成,而不是N SpaA 底物的识别。定量测量表明,Cd SrtA Δ生成的交联产物的催化周转数为 1.4 ± 0.004 h –1,其N SpaA 和肽底物的表观K M值为 0.16 ± 0.04 和 1.6 ± 0.3 mM ,分别。Cd SrtA Δ的活性比之前研究的变体高 7 倍,可在 6 小时内用肽标记 > 90% 的N SpaA。这项研究的结果进一步提高了Cd SrtA 作为蛋白质标记工具的实用性,并提供了对支撑蛋白质标记和菌毛生物发生的酶催化反应的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号