当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, Structure-Activity Relationship and Docking Studies of Novel Functionalized Arylvinyl-1,2,4-Trioxanes as Potent Antiplasmodial as well as Anticancer Agents.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-11 , DOI: 10.1002/cmdc.202000045 Mohit K Tiwari 1 , Paolo Coghi 2 , Prakhar Agrawal 3 , Bharti Rajesh K Shyamlal 1 , Li Jun Yang 4 , Lalit Yadav 1 , Yuzhong Peng 2 , Richa Sharma 1 , Dharmendra K Yadav 5 , Dinkar Sahal 3 , Vincent Kam Wai Wong 4 , Sandeep Chaudhary 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-11 , DOI: 10.1002/cmdc.202000045 Mohit K Tiwari 1 , Paolo Coghi 2 , Prakhar Agrawal 3 , Bharti Rajesh K Shyamlal 1 , Li Jun Yang 4 , Lalit Yadav 1 , Yuzhong Peng 2 , Richa Sharma 1 , Dharmendra K Yadav 5 , Dinkar Sahal 3 , Vincent Kam Wai Wong 4 , Sandeep Chaudhary 1
Affiliation
|
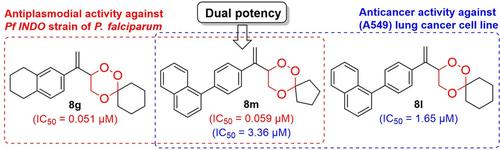
A novel series of synthetic functionalized arylvinyl‐1,2,4‐trioxanes (8 a –p ) has been prepared and assessed for their in vitro antiplasmodial activity against the chloroquine‐resistant Pf INDO strain of Plasmodium falciparum by using a SYBR green‐I fluorescence assay. Compounds 8 g (IC50=0.051 μM; SI=589.41) and 8 m (IC50=0.059 μM; SI=55.93) showed 11‐fold and >9‐fold more potent antiplasmodial activity, respectively, as compared to chloroquine (IC50=0.546 μM; SI=36.63). Different in silico docking studies performed on many target proteins revealed that the most active arylvinyl‐1,2,4‐trioxanes (8 g and 8 m ) showed dihydrofolate reductase (DHFR) binding affinities on a par with those of chloroquine and artesunate. The in vitro cytotoxic potentials of 8 a –p were also evaluated against human lung (A549) and liver (HepG2) cancer cell lines along with immortalized normal lung (BEAS‐2B) and liver (LO2) cell lines. Following screening, five derivatives viz. 8 a , 8 h , 8 l , 8 m and 8 o (IC50=1.65–31.7 μM; SI=1.08–10.96) were found to show potent cytotoxic activity against (A549) lung cancer cell lines, with selectivity superior to that of the reference compounds artemisinin (IC50=100 μM), chloroquine (IC50=100 μM) and artesunic acid (IC50=9.85 μM; SI=0.76). In fact, the most active 4‐naphthyl‐substituted analogue 8 l (IC50=1.65 μM; SI >10) exhibited >60 times more cytotoxicity than the standard reference, artemisinin, against A549 lung cancer cell lines. In silico docking studies of the most active anticancer compounds, 8 l and 8 m , against EGFR were found to validate the wet lab results. In summary, a new series of functionalized aryl‐vinyl‐1,2,4‐trioxanes (8 a –p ) has been shown to display dual potency as promising antiplasmodial and anticancer agents.
中文翻译:

新型功能化芳基乙烯基1,2,4-三恶烷作为有效的抗疟原虫药和抗癌药的设计,合成,结构活性关系和对接研究。
制备了一系列新的合成的功能化芳基乙烯基1,2,4-三恶烷(8 a – p),并使用SYBR green-I评估了它们对恶性疟原虫耐氯喹Pf INDO菌株的体外抗疟原虫活性。荧光测定。与氯喹(IC)相比,化合物8 g(IC 50 = 0.051μM; SI = 589.41)和8 m(IC 50 = 0.059μM ; SI = 55.93)分别显示出更强的抗血浆活性11倍和> 9倍50 = 0.546μM; SI = 36.63)。不同的计算机对许多靶蛋白的对接研究表明,活性最高的芳基乙烯基1,2,4-三恶烷(8 g和8 m)显示出二氢叶酸还原酶(DHFR)的结合亲和力与氯喹和青蒿琥酯的亲和力相当。在体外的细胞毒性潜力8 - p也针对人肺(A549)和肝(HepG2细胞)肿瘤细胞系评价与永生化的正常肺(BEAS-2B)和肝(LO2)细胞系一起。筛选后,得到五种衍生物。8 a,8 h,8 l,8 m和8 o(IC 50= 1.65–31.7μM;SI = 1.08–10.96)被发现对(A549)肺癌细胞系具有强大的细胞毒活性,其选择性优于参考化合物青蒿素(IC 50 = 100μM),氯喹(IC 50 = 100μM)和青蒿琥酯(IC 50=9.85μM; SI = 0.76)。事实上,最活跃的4-萘基取代类似物8 l(IC 50 = 1.65μM; SI> 10)对标准品青蒿素对A549肺癌细胞系的细胞毒性高出60倍以上。在计算机对接研究中,最活跃的抗癌化合物分别为8 l和8 m发现针对EGFR的,可以验证湿实验室的结果。总而言之,一系列新功能化的芳基-乙烯基1,2,4-三恶烷(8 a – p)已显示出作为有前途的抗血浆和抗癌药的双重功效。
更新日期:2020-07-03
中文翻译:

新型功能化芳基乙烯基1,2,4-三恶烷作为有效的抗疟原虫药和抗癌药的设计,合成,结构活性关系和对接研究。
制备了一系列新的合成的功能化芳基乙烯基1,2,4-三恶烷(8 a – p),并使用SYBR green-I评估了它们对恶性疟原虫耐氯喹Pf INDO菌株的体外抗疟原虫活性。荧光测定。与氯喹(IC)相比,化合物8 g(IC 50 = 0.051μM; SI = 589.41)和8 m(IC 50 = 0.059μM ; SI = 55.93)分别显示出更强的抗血浆活性11倍和> 9倍50 = 0.546μM; SI = 36.63)。不同的计算机对许多靶蛋白的对接研究表明,活性最高的芳基乙烯基1,2,4-三恶烷(8 g和8 m)显示出二氢叶酸还原酶(DHFR)的结合亲和力与氯喹和青蒿琥酯的亲和力相当。在体外的细胞毒性潜力8 - p也针对人肺(A549)和肝(HepG2细胞)肿瘤细胞系评价与永生化的正常肺(BEAS-2B)和肝(LO2)细胞系一起。筛选后,得到五种衍生物。8 a,8 h,8 l,8 m和8 o(IC 50= 1.65–31.7μM;SI = 1.08–10.96)被发现对(A549)肺癌细胞系具有强大的细胞毒活性,其选择性优于参考化合物青蒿素(IC 50 = 100μM),氯喹(IC 50 = 100μM)和青蒿琥酯(IC 50=9.85μM; SI = 0.76)。事实上,最活跃的4-萘基取代类似物8 l(IC 50 = 1.65μM; SI> 10)对标准品青蒿素对A549肺癌细胞系的细胞毒性高出60倍以上。在计算机对接研究中,最活跃的抗癌化合物分别为8 l和8 m发现针对EGFR的,可以验证湿实验室的结果。总而言之,一系列新功能化的芳基-乙烯基1,2,4-三恶烷(8 a – p)已显示出作为有前途的抗血浆和抗癌药的双重功效。







































 京公网安备 11010802027423号
京公网安备 11010802027423号