当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Infrared Spectrum of the Adamantane+ -Water Cation: Hydration-Induced C-H Bond Activation and Free Internal Water Rotation.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-11 , DOI: 10.1002/anie.202003637 Martin Andreas Robert George 1 , Marko Förstel 1 , Otto Dopfer 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-11 , DOI: 10.1002/anie.202003637 Martin Andreas Robert George 1 , Marko Förstel 1 , Otto Dopfer 1
Affiliation
|
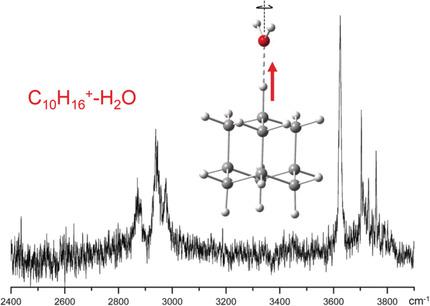
Diamondoid cations are reactive intermediates in their functionalization reactions in polar solution. Hydration is predicted to strongly activate their C−H bonds in initial proton abstraction reactions. To study the effects of microhydration on the properties of diamondoid cations, we characterize herein the prototypical monohydrated adamantane cation (C10H16+–H2O, Ad+–W) in its ground electronic state by infrared photodissociation spectroscopy in the CH and OH stretch ranges and dispersion‐corrected density functional theory (DFT) calculations. The water (W) ligand binds to the acidic CH group of Jahn–Teller distorted Ad+ via a strong CH⋅⋅⋅O ionic H‐bond supported by charge–dipole forces. Although W further enhances the acidity of this CH group along with a proton shift toward the solvent, the proton remains with Ad+ in the monohydrate. We infer essentially free internal W rotation from rotational fine structure of the ν3 band of W, resulting from weak angular anisotropy of the Ad+–W potential.
中文翻译:

金刚烷+-水阳离子的红外光谱:水合诱导的CH键活化和内部自由水旋转。
类金刚石阳离子是它们在极性溶液中的官能化反应中的反应性中间体。预计水合作用会在初始质子抽象反应中强烈激活其CH键。为了研究微水化对类金刚石阳离子性质的影响,我们在CH和红外光谱中通过红外光解光谱法表征了处于基态电子状态的典型一水合金刚烷阳离子(C 10 H 16 + –H 2 O,Ad + –W)。 OH拉伸范围和色散校正的密度泛函理论(DFT)计算。水(W)配体与Jahn–Teller扭曲的Ad +的酸性CH基结合通过电荷偶极力支持的强CH⋅⋅⋅O离子氢键。尽管W随着质子向溶剂的转移而进一步增强了该CH基的酸度,但质子与Ad +一起保留在一水合物中。我们推断从ν的旋转精细结构基本上不含,内部的W旋转3 W的频带,从广告的弱各向异性角度所得+ -W潜力。
更新日期:2020-07-06
中文翻译:

金刚烷+-水阳离子的红外光谱:水合诱导的CH键活化和内部自由水旋转。
类金刚石阳离子是它们在极性溶液中的官能化反应中的反应性中间体。预计水合作用会在初始质子抽象反应中强烈激活其CH键。为了研究微水化对类金刚石阳离子性质的影响,我们在CH和红外光谱中通过红外光解光谱法表征了处于基态电子状态的典型一水合金刚烷阳离子(C 10 H 16 + –H 2 O,Ad + –W)。 OH拉伸范围和色散校正的密度泛函理论(DFT)计算。水(W)配体与Jahn–Teller扭曲的Ad +的酸性CH基结合通过电荷偶极力支持的强CH⋅⋅⋅O离子氢键。尽管W随着质子向溶剂的转移而进一步增强了该CH基的酸度,但质子与Ad +一起保留在一水合物中。我们推断从ν的旋转精细结构基本上不含,内部的W旋转3 W的频带,从广告的弱各向异性角度所得+ -W潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号