Structure ( IF 4.4 ) Pub Date : 2020-05-12 , DOI: 10.1016/j.str.2020.04.006 Yasunori Watanabe 1 , Yasuo Watanabe 1 , Seiya Watanabe 2
|
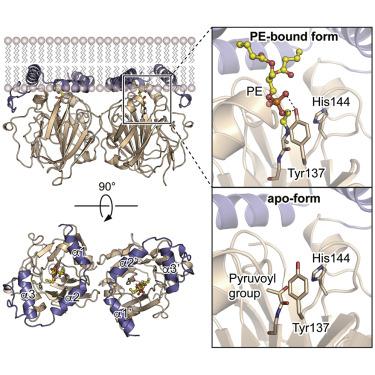
In both prokaryotes and eukaryotes, phosphatidylethanolamine (PE), one of the most abundant membrane phospholipids, plays important roles in various membrane functions and is synthesized through the decarboxylation of phosphatidylserine (PS) by PS decarboxylases (PSDs). However, the catalysis and substrate recognition mechanisms of PSDs remain unclear. In this study, we focused on the PSD from Escherichia coli (EcPsd) and determined the crystal structures of EcPsd in the apo form and PE-bound form at resolutions of 2.6 and 3.6 Å, respectively. EcPsd forms a homodimer, and each protomer has a positively charged substrate binding pocket at the active site. Structure-based mutational analyses revealed that conserved residues in the pocket are involved in PS decarboxylation. EcPsd has an N-terminal hydrophobic helical region that is important for membrane binding, thereby achieving efficient PS recognition. These results provide a structural basis for understanding the mechanism of PE biosynthesis by PSDs.
中文翻译:

细菌磷脂酰丝氨酸脱羧酶生物合成磷脂酰乙醇胺的结构基础。
在原核生物和真核生物中,磷脂酰乙醇胺(PE)是最丰富的膜磷脂之一,在各种膜功能中起着重要作用,并且是通过PS脱羧酶(PSD)通过磷脂酰丝氨酸(PS)的脱羧作用合成的。但是,PSD的催化和底物识别机制仍然不清楚。在这项研究中,我们专注于大肠杆菌的PSD(EcPsd),并分别以2.6和3.6Å的分辨率确定了apo形式和PE结合形式的EcPsd的晶体结构。EcPsd形成同型二聚体,每个启动子在活性位点均具有带正电的底物结合口袋。基于结构的突变分析表明,口袋中的保守残基与PS脱羧有关。EcPsd具有一个N端疏水性螺旋区域,该区域对于膜结合非常重要,从而实现了有效的PS识别。这些结果为了解PSDs合成PE的机理提供了结构基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号