Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-05-12 , DOI: 10.1016/j.jfluchem.2020.109548 Anjaneyulu Putta , Andrew G. Sykes , Haoran Sun
|
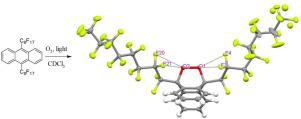
Aromatic endoperoxides serve as important intermediate species toward generating singlet state oxygen, which is key to photodynamic therapy applications. Gaining insights for fine-tuning endoperoxide stability and degree of oxygen-oxygen bond activation is of fundamental and practical interest for the chemical and medicinal communities. We report here that 910-bisperfluorooctyl-anthracene, upon exposing to light and air, is almost quantitatively converted to 910-bisperfluorooctyl-anthracene endoperoxide (compound 1) at room temperature. 1H NMR spectra and the X-ray crystal structure revealed that the resulting compound 1 is stable at room temperature without further decomposition. The crystal structure analysis showed that the compound 1 is stabilized by F···O intramolecular interactions along with F···F, F···H and F···C intermolecular interactions. DFT calculations further indicate that the degree of oxygen-oxygen bond activation in anthracene endoperoxides, reflected as changes of OO, C
O bond distances, may not solely depend on the electronic effect of substituents at the 910- positions. This uncertainty warrants further investigation both experimentally and computationally.
中文翻译:

全氟烷基内蒽过氧化物:合成,表征,晶体结构分析和计算洞察力
芳族过氧化物是产生单重态氧的重要中间物种,这是光动力疗法应用的关键。深入了解内过氧化物稳定性和氧-氧键活化程度的见解对于化学和医学界具有根本和实际意义。我们在此报告910-双全氟辛基-蒽在暴露于光和空气后,在室温下几乎被定量转化为910-双全氟辛基-蒽内过氧化物(化合物1)。1 H NMR光谱和X射线晶体结构表明,所得化合物1在室温下稳定,没有进一步分解。晶体结构分析表明化合物1通过F··O分子内相互作用以及F··F,F··H和F··C分子间相互作用来稳定。DFT计算进一步表明,蒽内过氧化物中氧-氧键活化的程度反映为O O,C
O键距离的变化,可能不仅仅取决于910-位上取代基的电子效应。这种不确定性值得进一步进行实验和计算研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号