Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-05-12 , DOI: 10.1016/j.bmc.2020.115549 Davana S Gonçalves 1 , Samara M de S Melo 1 , Andrey P Jacomini 1 , Michael J V da Silva 1 , Karlos E Pianoski 1 , Franciele Q Ames 2 , Rafael P Aguiar 2 , Alisson Felipe Oliveira 3 , Hélito Volpato 4 , Danielle L Bidóia 4 , Celso V Nakamura 4 , Ciomar A Bersani-Amado 2 , Davi F Back 5 , Sidnei Moura 6 , Fávero R Paula 3 , Fernanda A Rosa 1
|
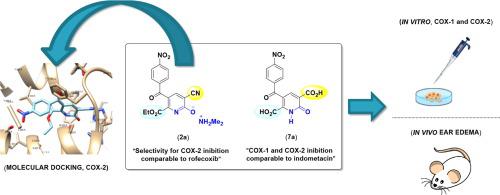
The inflammatory response is the reaction of living tissue to an injury of a foreign nature, such as infection and irritants, and occurs as part of the body's natural defence response. Compounds capable of inhibiting cyclooxygenase (COX) enzymes, especially COX-2, have great potential as anti-inflammatory agents. Herein we present the regioselective synthesis of 49 novel compounds based on the 2-pyridone nucleus. The topical anti-inflammatory activity of seventeen compounds was evaluated in mice by croton oil (CO) induced ear edema assay. Most of the compounds exhibited a high level of in vivo anti-inflammatory activity, reducing ear edema and myeloperoxidase (MPO) activity. The most active compounds (2a and 7a) were inhibitors of COX enzymes. Compound 2a selectively inhibited the COX-2, while 7a was nonselective. Further, the compound 2a showed effective binding at the active site of COX-2 co-crystal by docking molecular study.
中文翻译:

新型3,5,6-三取代2-吡啶酮衍生物的合成及其抗炎活性的评价。
炎症反应是活组织对异物损伤(如感染和刺激物)的反应,并作为人体自然防御反应的一部分而发生。能够抑制环氧合酶(COX)的化合物,尤其是COX-2,具有作为消炎药的巨大潜力。本文中,我们介绍了基于2-吡啶酮核的49种新型化合物的区域选择性合成。通过巴豆油(CO)诱导的耳部水肿试验评估了17种化合物在小鼠中的局部抗炎活性。大多数化合物表现出高水平的体内抗炎活性,减少了耳部水肿和髓过氧化物酶(MPO)的活性。活性最高的化合物(2a和7a)是COX酶的抑制剂。复合2a选择性抑制COX-2,而7a非选择性。此外,通过对接分子研究,化合物2a在COX-2共晶体的活性部位显示出有效结合。









































 京公网安备 11010802027423号
京公网安备 11010802027423号