当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dynamics and structural communication in the ternary complex of fully phosphorylated V2 vasopressin receptor, vasopressin, and β-arrestin 1.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-05-12 , DOI: 10.1016/j.bbamem.2020.183355 Luca Bellucci 1 , Angelo Felline 2 , Francesca Fanelli 3
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-05-12 , DOI: 10.1016/j.bbamem.2020.183355 Luca Bellucci 1 , Angelo Felline 2 , Francesca Fanelli 3
Affiliation
|
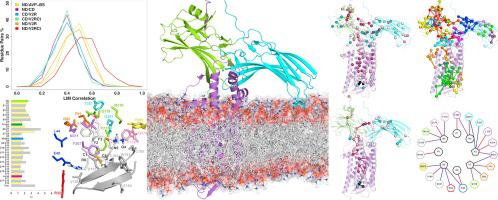
G protein-coupled receptors (GPCRs) are critically regulated by arrestins, which not only desensitize G-protein signaling but also initiate a G protein-independent wave of signaling. The information from structure determination was herein exploited to build a structural model of the ternary complex, comprising fully phosphorylated V2 vasopressin receptor (V2R), the agonist arginine vasopressin (AVP), and β-arrestin 1 (β-arr1). Molecular simulations served to explore dynamics and structural communication in the ternary complex. Flexibility and mechanical profiles reflect fold of V2R and β-arr1. Highly conserved amino acids tend to behave as hubs in the structure network and contribute the most to the mechanical rigidity of V2R seven-helix bundle and of β-arr1. Two structurally and dynamically distinct receptor-arrestin interfaces assist the twist of the N- and C-terminal domains (ND and CD, respectively) of β-arr1 with respect to each other, which is linked to arrestin activation. While motion of the ND is essentially assisted by the fully phosphorylated C-tail of V2R (V2RCt), that of CD is assisted by the second and third intracellular loops and the cytosolic extensions of helices 5 and 6. In the presence of the receptor, the β-arr1 inter-domain twist angle correlates with the modes describing the essential subspace of the ternary complex. β-arr1 motions are also influenced by the anchoring to the membrane of the C-edge-loops in the β-arr1-CD. Overall fluctuations reveal a coupling between motions of the agonist binding site and of β-arr1-ND, which are in allosteric communication between each other. Mechanical rigidity points, often acting as hubs in the structure network and distributed along the main axis of the receptor helix bundle, contribute to establish a preferential communication pathway between agonist ligand and the ND of arrestin. Such communication, mediated by highly conserved amino acids, involves also the first amino acid in the arrestin C-tail, which is highly dynamic and is involved in clathrin-mediated GPCR internalization.
中文翻译:

完全磷酸化的V2加压素受体,加压素和β-抑制蛋白三元复合物的动力学和结构连通性1。
G蛋白偶联受体(GPCR)受到抑制蛋白的严格调节,不仅使G蛋白信号脱敏,而且还引发了G蛋白非依赖性信号波。本文利用来自结构确定的信息来构建三元复合物的结构模型,该结构包含完全磷酸化的V2加压素受体(V2R),精氨酸加压素激动剂(AVP)和β-抑制蛋白1(β-arr1)。分子模拟有助于探索三元复合物中的动力学和结构通讯。柔韧性和机械性能反映了V2R和β-arr1的折叠。高度保守的氨基酸倾向于在结构网络中充当枢纽,并且对V2R七螺旋束和β-arr1的机械刚性贡献最大。两个结构上和动态上不同的受体-arrestin界面有助于β-arr1的N和C端结构域(分别为ND和CD)彼此之间的扭曲,这与抑制蛋白激活有关。尽管ND的运动基本上是由V2R(V2RCt)的完全磷酸化的C尾协助,而CD的运动则是由第二和第三胞内环以及螺旋5和6的胞质延伸协助。 β-arr1域间扭曲角与描述三元复合物基本子空间的模式相关。β-arr1的运动也受锚定在β-arr1-CD中C边缘环的膜上的影响。总体波动揭示了激动剂结合位点和β-arr1-ND的运动之间的耦合,它们之间是相互之间的变构通讯。机械刚性点通常在结构网络中充当枢纽,并沿着受体螺旋束的主轴分布,有助于在激动剂配体与抑制蛋白的ND之间建立优先的通讯途径。由高度保守的氨基酸介导的这种交流还涉及到了抑制蛋白C-tail中的第一个氨基酸,该氨基酸是高度动态的,并参与网格蛋白介导的GPCR内在化。
更新日期:2020-05-12
中文翻译:

完全磷酸化的V2加压素受体,加压素和β-抑制蛋白三元复合物的动力学和结构连通性1。
G蛋白偶联受体(GPCR)受到抑制蛋白的严格调节,不仅使G蛋白信号脱敏,而且还引发了G蛋白非依赖性信号波。本文利用来自结构确定的信息来构建三元复合物的结构模型,该结构包含完全磷酸化的V2加压素受体(V2R),精氨酸加压素激动剂(AVP)和β-抑制蛋白1(β-arr1)。分子模拟有助于探索三元复合物中的动力学和结构通讯。柔韧性和机械性能反映了V2R和β-arr1的折叠。高度保守的氨基酸倾向于在结构网络中充当枢纽,并且对V2R七螺旋束和β-arr1的机械刚性贡献最大。两个结构上和动态上不同的受体-arrestin界面有助于β-arr1的N和C端结构域(分别为ND和CD)彼此之间的扭曲,这与抑制蛋白激活有关。尽管ND的运动基本上是由V2R(V2RCt)的完全磷酸化的C尾协助,而CD的运动则是由第二和第三胞内环以及螺旋5和6的胞质延伸协助。 β-arr1域间扭曲角与描述三元复合物基本子空间的模式相关。β-arr1的运动也受锚定在β-arr1-CD中C边缘环的膜上的影响。总体波动揭示了激动剂结合位点和β-arr1-ND的运动之间的耦合,它们之间是相互之间的变构通讯。机械刚性点通常在结构网络中充当枢纽,并沿着受体螺旋束的主轴分布,有助于在激动剂配体与抑制蛋白的ND之间建立优先的通讯途径。由高度保守的氨基酸介导的这种交流还涉及到了抑制蛋白C-tail中的第一个氨基酸,该氨基酸是高度动态的,并参与网格蛋白介导的GPCR内在化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号