当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coincidence between Bond Strength, Atomic Abundance, and the Composition of Rocky Materials
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-05-12 , DOI: 10.1021/acsearthspacechem.0c00029 Edmund S. Doerksen 1 , Ryan C. Fortenberry 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-05-12 , DOI: 10.1021/acsearthspacechem.0c00029 Edmund S. Doerksen 1 , Ryan C. Fortenberry 1
Affiliation
|
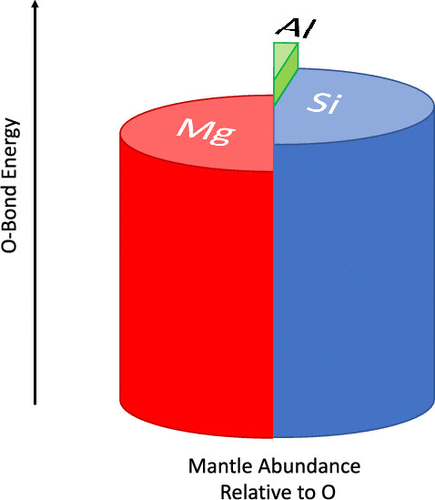
After removal the geologically and astrophysically underabundant Be, B, and F atoms from consideration, the strongest X–Y bonds in HnX–YHm hydrides are for O bonding with Al, Si, and Mg. These are stronger than the C–C bond in ethane or the C–O bond in methanol, for instance. These atoms, along with Fe, which is not a part of this study, are the dominant elements of rocky bodies and also happen to be the most common elements in the Earth’s lithosphere. This study has examined the bond strengths of all HnX–YHm hydrides from Li to Cl, except the noble gas Ne. Hence, this work indicates a possible link between simple hydrides, like HOAlH2, and the beginning stages of mineral nanocrystal formation in the early solar system or interstellar grain nucleation. These strong bonds likely arise from notably strong ionic interactions between the third-row atoms with oxygen and may drive the early condensation of refractory materials in astrophysical contexts.
中文翻译:

结合强度,原子丰度与岩石材料组成之间的符合
从地质和天体上去除了剩余的Be,B和F原子后,H n X-YH m氢化物中最强的X-Y键用于与Al,Si和Mg进行O键键合。例如,它们比乙烷中的C–C键或甲醇中的C–O键强。这些原子与铁(不是本研究的一部分)一起是岩石体的主要元素,也恰好是地球岩石圈中最常见的元素。这项研究研究了除稀有气体Ne以外的所有Li x Cl的H n X–YH m氢化物的结合强度。因此,这项工作表明简单氢化物之间可能存在联系,例如HOAlH 2,以及早期太阳系或星际晶粒成核过程中矿物纳米晶体形成的开始阶段。这些强键可能是由于第三行原子与氧气之间明显的离子相互作用而引起的,并可能导致天体物理学中耐火材料的早期凝结。
更新日期:2020-06-18
中文翻译:

结合强度,原子丰度与岩石材料组成之间的符合
从地质和天体上去除了剩余的Be,B和F原子后,H n X-YH m氢化物中最强的X-Y键用于与Al,Si和Mg进行O键键合。例如,它们比乙烷中的C–C键或甲醇中的C–O键强。这些原子与铁(不是本研究的一部分)一起是岩石体的主要元素,也恰好是地球岩石圈中最常见的元素。这项研究研究了除稀有气体Ne以外的所有Li x Cl的H n X–YH m氢化物的结合强度。因此,这项工作表明简单氢化物之间可能存在联系,例如HOAlH 2,以及早期太阳系或星际晶粒成核过程中矿物纳米晶体形成的开始阶段。这些强键可能是由于第三行原子与氧气之间明显的离子相互作用而引起的,并可能导致天体物理学中耐火材料的早期凝结。











































 京公网安备 11010802027423号
京公网安备 11010802027423号