Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-05-12 , DOI: 10.1016/j.omtm.2020.05.001 Benjamin J Samelson-Jones 1, 2, 3 , Jonathan D Finn 1 , Patricia Favaro 1 , J Fraser Wright 1, 3 , Valder R Arruda 1, 2, 3
|
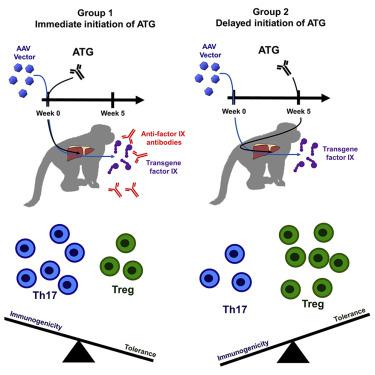
Adeno-associated virus (AAV) vector gene therapy is as a promising treatment for a variety of genetic diseases, including hemophilia. Systemic administration of AAV vectors is associated with a cytotoxic immune response triggered against AAV capsid proteins, which if untreated can result in loss of transgene expression. Immunosuppression (IS) with corticosteroids has limited transgene loss in some AAV gene therapy clinical trials, but was insufficient to prevent loss in other studies. We used a nonhuman primate model to evaluate intensive T cell directed IS combined with AAV-mediated transfer of the human factor IX (FIX) gene. Early administration of rabbit antithymocyte globulin (ATG) concomitant with AAV administration resulted in the development of anti-FIX antibodies while delayed ATG by 5 weeks administration did not. The anti-FIX immune response was associated with increases in inflammatory cytokines as well as a skewed Th17/Treg ratio. We conclude that the timing of T cell directed IS is critical in determining transgene-product immunogenicity or tolerance. These data have implications for systemically administered AAV gene therapy being evaluated for hemophilia A and B as well as other genetic diseases. In nonhuman primates, Samelson-Jones et al. observe that early intensive immunosuppression concomitant with AAV-vector administration enhance the formation of transgene-product antibodies while delaying immunosuppression by 5 weeks does not. This suggests there is a critical time-period when intensive immunosuppression can shift the immune system towards immunogenicity and away from tolerance.
中文翻译:

强化免疫抑制的时机会影响非人灵长类动物 AAV 基因治疗后转基因抗体的风险。
腺相关病毒(AAV)载体基因疗法是治疗包括血友病在内的多种遗传性疾病的一种有前途的治疗方法。 AAV 载体的全身给药与针对 AAV 衣壳蛋白触发的细胞毒性免疫反应有关,如果不进行处理,可能会导致转基因表达丧失。在一些 AAV 基因治疗临床试验中,皮质类固醇的免疫抑制 (IS) 限制了转基因损失,但在其他研究中不足以防止损失。我们使用非人灵长类动物模型来评估强化 T 细胞定向 IS 与 AAV 介导的人类因子 IX (FIX) 基因转移相结合。早期给予兔抗胸腺细胞球蛋白 (ATG) 并同时给予 AAV 会导致抗 FIX 抗体的产生,而延迟 ATG 5 周给药则不会。抗 FIX 免疫反应与炎症细胞因子的增加以及 Th17/Treg 比率的倾斜有关。我们得出的结论是,T 细胞定向 IS 的时机对于确定转基因产物的免疫原性或耐受性至关重要。这些数据对于评估A型和B型血友病以及其他遗传病的系统性AAV基因治疗具有重要意义。在非人类灵长类动物中,Samelson-Jones 等人。观察到,与 AAV 载体给药同时进行的早期强化免疫抑制可增强转基因产物抗体的形成,而延迟免疫抑制 5 周则不会。这表明存在一个关键时期,强化免疫抑制可以使免疫系统转向免疫原性并远离耐受性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号