当前位置:
X-MOL 学术
›
Acta Cryst. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Supramolecular structure, in vivo biological activities and molecular-docking-based potential cardiotoxic exploration of aconine hydrochloride monohydrate as a novel salt form.
Acta Crystallographica Section B ( IF 1.3 ) Pub Date : 2020-03-23 , DOI: 10.1107/s2052520620001250 Han Qing Li 1 , Jia Yin Xu 2 , Yuan Yuan Gao 1 , Liang Jin 1 , Jian Ming Chen 3 , Feng Zheng Chen 4
Acta Crystallographica Section B ( IF 1.3 ) Pub Date : 2020-03-23 , DOI: 10.1107/s2052520620001250 Han Qing Li 1 , Jia Yin Xu 2 , Yuan Yuan Gao 1 , Liang Jin 1 , Jian Ming Chen 3 , Feng Zheng Chen 4
Affiliation
|
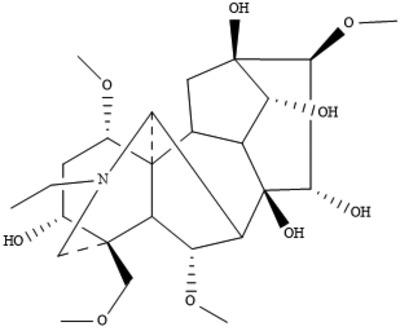
Despite the high profile of aconine in WuTou injection, there has been no preparative technology or structural studies of its salt as the pharmaceutical product. The lack of any halide salt forms is surprising as aconine contains a tertiary nitrogen atom. In this work, aconine was prepared from the degradation of aconitine in Aconiti kusnezoffii radix (CaoWu). A green chemistry technique was applied to enrich the lipophilic‐poor aconine. Reaction of aconine with hydrochloride acid resulted in protonation of the nitrogen atom and gave a novel salt form (C25H42NO9+·Cl−·H2O; aconine hydrochloride monohydrate, AHM), whose cation in the crystal structure was elucidated based on extensive spectroscopic and X‐ray crystallographic analyses. The AHM crystal had a Z′ = 3 structure with three independent cation–anion pairs, with profound conformational differences among the aconine cations. The central framework of each aconine cation was compared with that of previously reported aconitine, proving that protonation of the nitrogen atom induced the structure rearrangement. In the crystal of AHM, aconine cations, chloride anions and water molecules interacted through inter‐species O—H…Cl and O—H…O hydrogen bonds; this complex hydrogen‐bonding network stabilizes the supramolecular structure. The seriously disordered solvent molecules were treated using the PLATON SQUEEZE procedure [Spek (2015). Acta Cryst. C71, 9–18] and their atoms were therefore omitted from the refinement. Bioactivity studies indicated that AHM promoted in vitro proliferative activities of RAW264.7 cells. Molecular docking suggested AHM could target cardiotoxic protein through the hydrogen‐bonding interactions. The structural confirmation of AHM offers a rational approach for improving the pharmaceutical technology of WuTou injection.
中文翻译:

乌头碱盐酸盐一水合物作为新型盐形式的超分子结构,体内生物活性和基于分子对接的潜在心脏毒性探索。
尽管乌头注射液中乌头碱含量很高,但尚无有关其盐类药物的制备技术或结构研究。缺少任何卤化物盐形式是令人惊讶的,因为乌头碱含有叔氮原子。在这项工作中,乌头碱是由乌头草乌头中乌头碱的降解制备的。应用绿色化学技术富集了亲脂性差的乌头碱。用盐酸乌头原碱的反应导致的氮原子的质子化并给予了新的盐形式(C 25 ħ 42 NO 9 + ·氯- ·H 2O; 盐酸乌头碱一水合物(AHM),其晶体结构中的阳离子已通过广泛的光谱和X射线晶体学分析得以阐明。AHM晶体具有Z ′= 3结构,具有三个独立的阳离子-阴离子对,乌头碱阳离子之间构型差异很大。将每个乌头碱阳离子的中心构架与先前报道的乌头碱进行了比较,证明了氮原子的质子化诱导了结构重排。在AHM的晶体中,乌头碱阳离子,氯离子和水分子通过种间的OH-Cl和OH-O相互作用。这个复杂的氢键网络稳定了超分子结构。使用PLATON处理了严重无序的溶剂分子挤压程序[Spek(2015)。Acta Cryst。C 71 [ 9-18]及其原子因此被从精制中省略。生物活性研究表明,AHM可促进RAW264.7细胞的体外增殖活性。分子对接表明,AHM可以通过氢键相互作用靶向心脏毒性蛋白。AHM的结构验证为改善五头注射液的制药工艺提供了合理的途径。
更新日期:2020-03-23
中文翻译:

乌头碱盐酸盐一水合物作为新型盐形式的超分子结构,体内生物活性和基于分子对接的潜在心脏毒性探索。
尽管乌头注射液中乌头碱含量很高,但尚无有关其盐类药物的制备技术或结构研究。缺少任何卤化物盐形式是令人惊讶的,因为乌头碱含有叔氮原子。在这项工作中,乌头碱是由乌头草乌头中乌头碱的降解制备的。应用绿色化学技术富集了亲脂性差的乌头碱。用盐酸乌头原碱的反应导致的氮原子的质子化并给予了新的盐形式(C 25 ħ 42 NO 9 + ·氯- ·H 2O; 盐酸乌头碱一水合物(AHM),其晶体结构中的阳离子已通过广泛的光谱和X射线晶体学分析得以阐明。AHM晶体具有Z ′= 3结构,具有三个独立的阳离子-阴离子对,乌头碱阳离子之间构型差异很大。将每个乌头碱阳离子的中心构架与先前报道的乌头碱进行了比较,证明了氮原子的质子化诱导了结构重排。在AHM的晶体中,乌头碱阳离子,氯离子和水分子通过种间的OH-Cl和OH-O相互作用。这个复杂的氢键网络稳定了超分子结构。使用PLATON处理了严重无序的溶剂分子挤压程序[Spek(2015)。Acta Cryst。C 71 [ 9-18]及其原子因此被从精制中省略。生物活性研究表明,AHM可促进RAW264.7细胞的体外增殖活性。分子对接表明,AHM可以通过氢键相互作用靶向心脏毒性蛋白。AHM的结构验证为改善五头注射液的制药工艺提供了合理的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号