Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-05-11 , DOI: 10.1016/j.jaci.2020.03.046 Shruti Rastogi 1 , Diana Maria Willmes 1 , Maria Nassiri 1 , Magda Babina 1 , Margitta Worm 1
|
Background
Reduced levels of prostaglandin E2 (PGE2) contribute to aspirin-induced hypersensitivity. COX inhibitors are also frequent cofactors in anaphylaxis. Whether alterations in the PGE2 system contribute to anaphylaxis independently of COX inhibitor intake is unclear.
Objective
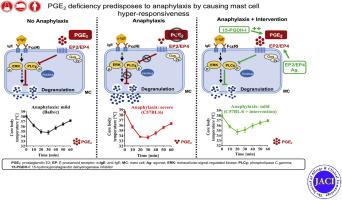
Our aim was to test the hypothesis that relative PGE2 deficiency predisposes to anaphylaxis.
Methods
Sera from 48 patients with anaphylaxis and 27 healthy subjects were analyzed for PGE2 levels and correlated against severity; 9α,11β-PGF2 and PGI2 metabolites were measured for control purposes. PGE2 stabilization by 15-hydroxyprostaglandin dehydrogenase inhibitor or EP2 or EP4 receptor agonists were used in a murine model of passive systemic anaphylaxis. FcεRI-triggered mediator release was determined in bone marrow–derived cultured mast cells (MCs) and human skin–derived MCs. Signaling was studied by Western blot analysis.
Results
Patients with anaphylaxis were characterized by markedly reduced PGE2 levels vis-à-vis healthy subjects, whereas prostacyclin metabolite levels were diminished only weakly, and 9α,11β-PGF2 levels conversely increased. PGE2 was negatively correlated with severity. Lower PGE2 levels and higher susceptibility to anaphylaxis were also found in C57BL/6 mice vis-à-vis in Balb/c mice. Stabilization of PGE2 level by 15-hydroxyprostaglandin dehydrogenase inhibitor protected mice against anaphylaxis. Exogenous PGE2 attenuated bone marrow–derived cultured MC activation through EP2 and EP4 receptors. EP2 and EP4 agonism also curbed FcεRI-mediated degranulation of human MCs. Mechanistically, PGE2 interfered with the phosphorylation of phospholipase C gamma-1 and extracellular signal–regulated kinase.
Conclusions
Homeostatic levels of PGE2 attenuate MC activation via EP2/EP4 and protect against anaphylaxis. Relative deficiency of PGE2 predisposes to anaphylaxis in humans and mice, whereas PGE2 stabilization protects against anaphylactic reactions.
中文翻译:

PGE2缺乏会导致肥大细胞高反应性,从而导致过敏反应。
背景
降低的前列腺素E 2(PGE 2)水平有助于阿司匹林引起的超敏反应。COX抑制剂也是过敏反应的常见辅助因子。目前尚不清楚PGE 2系统的改变是否独立于COX抑制剂的摄入而导致过敏反应。
目的
我们的目的是检验相对PGE 2缺乏易感的假说。
方法
分析了来自48名过敏反应患者和27名健康受试者的血清中PGE 2的水平,并与严重程度相关。9α,11β-PGF 2和PGI 2种用于控制目的,测量代谢物。15-羟基前列腺素脱氢酶抑制剂或EP2或EP4受体激动剂对PGE 2的稳定作用被用于被动全身性过敏反应的小鼠模型中。FcεRI触发的介质释放是在源自骨髓的培养的肥大细胞(MC)和源自人体皮肤的MC中确定的。通过蛋白质印迹分析研究信号传导。
结果
通过显着减少PGE患者过敏性反应进行了表征2水平VIS-à-VIS健康受试者,而前列环素代谢物水平均减弱只有弱,和9α,11β-PGF 2水平反过来增加。PGE 2与严重程度呈负相关。与Balb / c小鼠相比,C57BL / 6小鼠中还发现较低的PGE 2水平和较高的过敏反应敏感性。15-羟基前列腺素脱氢酶抑制剂稳定PGE 2水平可保护小鼠免于过敏反应。外源PGE 2通过EP2和EP4受体减弱了源自骨髓的培养MC激活。EP2和EP4激动剂还可以抑制FcεRI介导的人MC脱粒。从机理上讲,PGE 2干扰磷脂酶C gamma-1和细胞外信号调节激酶的磷酸化。
结论
PGE 2的体内稳态水平可通过EP2 / EP4减弱MC激活,并防止过敏反应。PGE 2相对缺乏会导致人类和小鼠过敏反应,而PGE 2稳定化可防止过敏反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号