Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-05-11 , DOI: 10.1016/j.jaci.2020.03.044 Karin Hartmann 1 , Jason Gotlib 2 , Cem Akin 3 , Olivier Hermine 4 , Farrukh T Awan 5 , Elizabeth Hexner 6 , Michael J Mauro 7 , Hans D Menssen 8 , Suman Redhu 8 , Stefanie Knoll 9 , Karl Sotlar 10 , Tracy I George 11 , Hans-Peter Horny 12 , Peter Valent 13 , Andreas Reiter 14 , Hanneke C Kluin-Nelemans 15
|
Background
Advanced systemic mastocytosis (advSM) is characterized by presence of the KIT D816V mutation and pathologic accumulation of neoplastic mast cells (MCs) in various tissues, leading to severe symptoms and organ damage (eg, cytopenias, liver dysfunction, portal hypertension, malabsorption, and weight loss). Treatment with midostaurin, an orally active multikinase/KIT inhibitor now approved for advSM in the United States and the European Union, resulted in a high rate of response accompanied by reduced MC infiltration of the bone marrow and lowered serum tryptase level.
Objective
We aimed to determine whether midostaurin improves health-related quality of life (QOL) and MC mediator–related symptoms in patients with advSM.
Methods
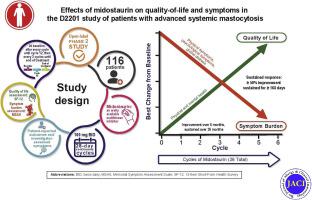
In 116 patients with systemic mastocytosis (89 patients with advSM fulfilling the strict inclusion criteria of the D2201 study [ClinicalTrials.gov identifier NCT00782067]), QOL and symptom burden were assessed during treatment with midostaurin by using the 12-Item Short-Form Health Survey (SF-12) and the Memorial Symptom Assessment Scale patient-reported questionnaires, respectively. MC mediator–related symptoms were evaluated by using a specific physician-reported questionnaire.
Results
Over the first 6 cycles of treatment with midostaurin (ie, 6 months), patients experienced significant improvements in total SF-12 and Memorial Symptom Assessment Scale scores, as well as in subscores of each instrument. These improvements were durable during 36 months of follow-up. Similarly, we found substantial improvements (67%-100%) in all MC mediator–related symptoms.
Conclusion
QOL and MC mediator–related symptoms significantly improve with midostaurin treatment in patients with advSM (ClinicalTrials.gov identifier, NCT00782067).
中文翻译:

Midostaurin可改善晚期全身性肥大细胞增多症的生活质量和与介体有关的症状。
背景
晚期全身肥大细胞增多症(advSM)的特征是在各种组织中都存在KIT D816V突变和肿瘤性肥大细胞(MCs)的病理积累,从而导致严重的症状和器官损害(例如,血细胞减少,肝功能不全,门脉高压,吸收不良和减肥)。Midostaurin是一种口服活性多激酶/ KIT抑制剂,目前已在美国和欧盟批准用于advSM的治疗,导致反应率高,同时骨髓MC浸润减少,血清类胰蛋白酶水平降低。
目的
我们旨在确定Midostaurin是否能改善advSM患者的健康相关生活质量(QOL)和MC介体相关症状。
方法
在116例系统性肥大细胞增多症患者中(89例advSM患者符合D2201研究的严格纳入标准[ClinicalTrials.gov标识符NCT00782067]),在米ostaurin治疗期间通过使用12项简短形式健康调查评估了生活质量和症状负担(SF-12)和纪念症状评估量表的患者报告问卷。通过使用医生报告的特定问卷评估与MC介体有关的症状。
结果
在米多骨蛋白治疗的前6个周期(即6个月)中,患者的SF-12总评分和纪念症状评估量表评分以及每种器械的亚评分均显着提高。这些改善在随访的36个月内是持久的。同样,我们发现所有与MC介体有关的症状都有实质性改善(67%-100%)。
结论
在advSM患者中,用Midostaurin治疗可明显改善QOL和MC介体相关的症状(ClinicalTrials.gov标识符,NCT00782067)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号