Cell Stem Cell ( IF 19.8 ) Pub Date : 2020-05-11 , DOI: 10.1016/j.stem.2020.04.017 Haibin Xi 1 , Justin Langerman 2 , Shan Sabri 2 , Peggie Chien 3 , Courtney S Young 4 , Shahab Younesi 1 , Michael Hicks 1 , Karen Gonzalez 5 , Wakana Fujiwara 6 , Julia Marzi 7 , Simone Liebscher 8 , Melissa Spencer 9 , Ben Van Handel 10 , Denis Evseenko 10 , Katja Schenke-Layland 11 , Kathrin Plath 12 , April D Pyle 13
|
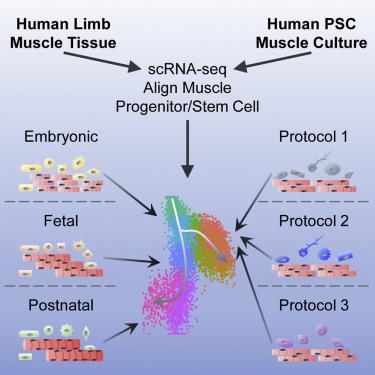
The developmental trajectory of human skeletal myogenesis and the transition between progenitor and stem cell states are unclear. We used single-cell RNA sequencing to profile human skeletal muscle tissues from embryonic, fetal, and postnatal stages. In silico, we identified myogenic as well as other cell types and constructed a “roadmap” of human skeletal muscle ontogeny across development. In a similar fashion, we also profiled the heterogeneous cell cultures generated from multiple human pluripotent stem cell (hPSC) myogenic differentiation protocols and mapped hPSC-derived myogenic progenitors to an embryonic-to-fetal transition period. We found differentially enriched biological processes and discovered co-regulated gene networks and transcription factors present at distinct myogenic stages. This work serves as a resource for advancing our knowledge of human myogenesis. It also provides a tool for a better understanding of hPSC-derived myogenic progenitors for translational applications in skeletal muscle-based regenerative medicine.
中文翻译:

人类骨骼肌图谱确定了干细胞和祖细胞在发育过程中以及来自人类多能干细胞的轨迹。
人类骨骼肌生成的发展轨迹以及祖细胞和干细胞状态之间的转变尚不清楚。我们使用单细胞 RNA 测序来分析胚胎、胎儿和产后阶段的人类骨骼肌组织。在计算机中, 我们确定了生肌细胞和其他细胞类型,并构建了人类骨骼肌个体发育的“路线图”。以类似的方式,我们还分析了从多个人类多能干细胞 (hPSC) 成肌分化方案产生的异质细胞培养物,并将 hPSC 衍生的成肌祖细胞映射到胚胎到胎儿的过渡期。我们发现了差异丰富的生物过程,并发现了存在于不同生肌阶段的共同调节基因网络和转录因子。这项工作可作为提高我们对人类肌生成知识的资源。它还提供了一种工具,可以更好地了解 hPSC 衍生的肌源性祖细胞在基于骨骼肌的再生医学中的转化应用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号