当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical synthesis of human syndecan-4 glycopeptide bearing O-, N-sulfation and multiple aspartic acids for probing impacts of the glycan chain and the core peptide on biological functions
Chemical Science ( IF 7.6 ) Pub Date : 2020-05-11 , DOI: 10.1039/d0sc01140a Weizhun Yang 1 , Yigitcan Eken 1 , Jicheng Zhang 1 , Logan Emerson Cole 1 , Sherif Ramadan 1, 2 , Yongmei Xu 3 , Zeren Zhang 1 , Jian Liu 3 , Angela K Wilson 1 , Xuefei Huang 1, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020-05-11 , DOI: 10.1039/d0sc01140a Weizhun Yang 1 , Yigitcan Eken 1 , Jicheng Zhang 1 , Logan Emerson Cole 1 , Sherif Ramadan 1, 2 , Yongmei Xu 3 , Zeren Zhang 1 , Jian Liu 3 , Angela K Wilson 1 , Xuefei Huang 1, 4, 5
Affiliation
|
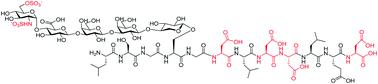
Proteoglycans are a family of complex glycoproteins with glycosaminoglycan chains such as heparan sulfate (HS) attached to the core protein backbone. Due to the high structural heterogeneity of HS in nature, it is challenging to decipher the respective roles of the HS chain and the core protein on proteoglycan functions. While the sulfation patterns of HS dictate many activities, the core protein can potentially impact HS functions. In order to decipher this, homogeneous proteoglycan glycopeptides are needed. Herein, we report the first successful synthesis of proteoglycan glycopeptides bearing multiple aspartic acids in the core peptide and O- and N-sulfations in the glycan chain, as exemplified by the syndecan-4 glycopeptides. To overcome the high acid sensitivities of sulfates and base sensitivities of the glycopeptide during synthesis, a new synthetic approach has been developed to produce a sulfated glycan chain on a peptide sequence prone to the formation of aspartimide side products. The availability of the structurally well-defined synthetic glycopeptide enabled the investigation of their biological functions including cytokine, growth factor binding and heparanase inhibition. Interestingly, the glycopeptide exhibited context dependent enhancement or decrease of biological activities compared to the peptide or the glycan alone. The results presented herein suggest that besides varying the sulfation patterns of HS, linking the HS chain to core proteins as in proteoglycans may be an additional approach to modulate biological functions of HS in nature.
中文翻译:

化学合成带有 O-、N- 硫酸化和多种天冬氨酸的人 syndecan-4 糖肽,用于探讨聚糖链和核心肽对生物学功能的影响
蛋白聚糖是一类复杂的糖蛋白,其糖胺聚糖链如硫酸乙酰肝素 (HS) 连接到核心蛋白主链上。由于 HS 在自然界中的高度结构异质性,破译 HS 链和核心蛋白对蛋白多糖功能的各自作用具有挑战性。虽然 HS 的硫酸化模式决定了许多活动,但核心蛋白可能会影响 HS 功能。为了破译这一点,需要均质的蛋白聚糖糖肽。在此,我们报告了在核心肽中带有多个天冬氨酸并在聚糖链中带有 O- 和 N- 硫酸盐的蛋白多糖糖肽的首次成功合成,例如 syndecan-4 糖肽。为了克服合成过程中硫酸盐的高酸敏感性和糖肽的碱敏感性,已开发出一种新的合成方法,可在易于形成阿斯巴酰亚胺副产物的肽序列上产生硫酸化聚糖链。结构明确的合成糖肽的可用性使得能够研究其生物学功能,包括细胞因子、生长因子结合和乙酰肝素酶抑制。有趣的是,与单独的肽或聚糖相比,糖肽表现出依赖于上下文的生物活性增强或降低。本文提供的结果表明,除了改变 HS 的硫酸化模式之外,将 HS 链连接到蛋白聚糖中的核心蛋白可能是调节 HS 在自然界中的生物学功能的另一种方法。
更新日期:2020-07-01
中文翻译:

化学合成带有 O-、N- 硫酸化和多种天冬氨酸的人 syndecan-4 糖肽,用于探讨聚糖链和核心肽对生物学功能的影响
蛋白聚糖是一类复杂的糖蛋白,其糖胺聚糖链如硫酸乙酰肝素 (HS) 连接到核心蛋白主链上。由于 HS 在自然界中的高度结构异质性,破译 HS 链和核心蛋白对蛋白多糖功能的各自作用具有挑战性。虽然 HS 的硫酸化模式决定了许多活动,但核心蛋白可能会影响 HS 功能。为了破译这一点,需要均质的蛋白聚糖糖肽。在此,我们报告了在核心肽中带有多个天冬氨酸并在聚糖链中带有 O- 和 N- 硫酸盐的蛋白多糖糖肽的首次成功合成,例如 syndecan-4 糖肽。为了克服合成过程中硫酸盐的高酸敏感性和糖肽的碱敏感性,已开发出一种新的合成方法,可在易于形成阿斯巴酰亚胺副产物的肽序列上产生硫酸化聚糖链。结构明确的合成糖肽的可用性使得能够研究其生物学功能,包括细胞因子、生长因子结合和乙酰肝素酶抑制。有趣的是,与单独的肽或聚糖相比,糖肽表现出依赖于上下文的生物活性增强或降低。本文提供的结果表明,除了改变 HS 的硫酸化模式之外,将 HS 链连接到蛋白聚糖中的核心蛋白可能是调节 HS 在自然界中的生物学功能的另一种方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号