当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
5-Hydroxymethylfurfural Synthesis in Nonaqueous Two-Phase Systems (NTPS)–PC-SAFT Predictions and Validation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.oprd.0c00072 Michael Knierbein 1 , Matthias Voges 1 , Christoph Held 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.oprd.0c00072 Michael Knierbein 1 , Matthias Voges 1 , Christoph Held 1
Affiliation
|
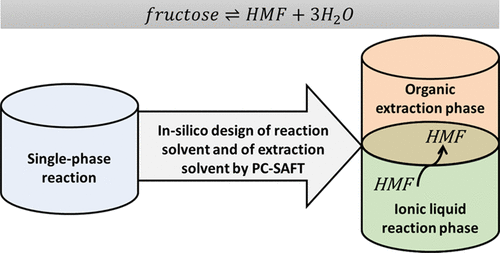
The condensation reaction of fructose to 5-hydroxymethylfurfural (HMF) is acid-catalyzed, and it suffers from thermodynamic limitation of the conversion, poor kinetics, and consecutive reactions such as formation of humins from HMF. Different approaches exist to overcome these limitations. In this work, the combination of a nonaqueous solvent and a suitable extraction system is presented that ensures high reaction selectivity at full conversion, fast kinetics, and high partition selectivity of the product HMF over reactant fructose, keeping the temperature as low as 70 °C. In the first step of this work, the equation of state PC-SAFT was used to predict solvent effects on the reaction equilibrium of homogenous reaction systems. It was found that the two hydrophilic ionic liquids (ILs) [BMIM]Cl and [MIM]Cl shifted the reaction equilibrium to the product side by factors of 230 and 40, respectively, compared to the reaction in water. The predictions were verified by experimental data, which showed full conversion of fructose to HMF within less than 20 (60) min for the reaction in [MIM]Cl ([BMIM]Cl) with a high selectivity of up to 80%. Even more, the reaction in the [MIM]Cl solvent did not require adding a catalyst due to the acidic character of this IL. In the second step of this work, an in situ extraction of HMF was performed using a nonaqueous two-phase reaction system NTPS that was designed with PC-SAFT. The NTPS contains the reaction phase (either [BMIM]Cl or [MIM]Cl) and the extraction agent, i.e., one of the ketones MEK, MIBK, or ethyl acetate. These IL + organic solvent NTPSs were analyzed and evaluated toward fructose conversion and partitioning of fructose and of HMF. PC-SAFT predicted that, among all systems studied in this work, the NTPS IL + MEK was the most promising for the reaction of fructose to HMF and the in situ removal of HMF from fructose. Experimental results could validate the PC-SAFT predictions, that is, IL + MEK NTPSs allowed efficient conversion of fructose to HMF and a partition selectivity of HMF over fructose of about 100%. This new NTPS does not require an additional catalyst due to the acidity of [MIM]Cl; it allows a high reaction selectivity of 87% at 20 min and 93% conversion, and it moreover provides high separation efficiency. In sum, these results open the door for further developments of in situ extraction systems in the future for efficient and fast fructose conversion and HMF separation from the reacting phase, keeping the temperature as low as possible.
中文翻译:

非水两相系统(NTPS)中的5-羟甲基糠醛合成–PC-SAFT预测和验证
果糖与5-羟甲基糠醛(HMF)的缩合反应是酸催化的,并且存在转化的热力学限制,动力学差以及诸如从HMF形成腐殖质等连续反应的问题。存在克服这些限制的不同方法。在这项工作中,提出了一种非水溶剂和合适的萃取系统的组合,可确保全转化时的高反应选择性,快速动力学和产物HMF在果糖上的高分配选择性,并保持温度低至70°C 。在这项工作的第一步中,状态方程PC-SAFT用于预测溶剂对均相反应体系反应平衡的影响。已发现,与在水中的反应相比,两种亲水性离子液体(ILs)[BMIM] Cl和[MIM] Cl分别将反应平衡向产物侧移动了230和40倍。通过实验数据验证了这些预测,实验数据显示果糖在[MIM] Cl([BMIM] Cl)中的反应在不到20(60)分钟内完全转化为HMF,选择性高达80%。甚至由于该IL的酸性特征,在[MIM] Cl溶剂中的反应不需要添加催化剂。在这项工作的第二步中,使用由PC-SAFT设计的非水两相反应系统NTPS对HMF进行原位提取。NTPS包含反应相([BMIM] Cl或[MIM] Cl)和萃取剂,即酮MEK,MIBK或乙酸乙酯之一。分析并评估了这些IL +有机溶剂NTPS的果糖转化率以及果糖和HMF的分配。PC-SAFT预测,在这项工作研究的所有系统中,对于果糖与HMF的反应以及从果糖中原位去除HMF而言,NTPS IL + MEK是最有前途的。实验结果可以验证PC-SAFT的预测,即IL + MEK NTPS可以将果糖有效地转化为HMF,并且HMF相对于果糖的分配选择性约为100%。由于[MIM] Cl的酸度,这种新的NTPS不需要额外的催化剂。它在20分钟时可实现87%的高反应选择性和93%的转化率,而且分离效率高。总共,
更新日期:2020-06-19
中文翻译:

非水两相系统(NTPS)中的5-羟甲基糠醛合成–PC-SAFT预测和验证
果糖与5-羟甲基糠醛(HMF)的缩合反应是酸催化的,并且存在转化的热力学限制,动力学差以及诸如从HMF形成腐殖质等连续反应的问题。存在克服这些限制的不同方法。在这项工作中,提出了一种非水溶剂和合适的萃取系统的组合,可确保全转化时的高反应选择性,快速动力学和产物HMF在果糖上的高分配选择性,并保持温度低至70°C 。在这项工作的第一步中,状态方程PC-SAFT用于预测溶剂对均相反应体系反应平衡的影响。已发现,与在水中的反应相比,两种亲水性离子液体(ILs)[BMIM] Cl和[MIM] Cl分别将反应平衡向产物侧移动了230和40倍。通过实验数据验证了这些预测,实验数据显示果糖在[MIM] Cl([BMIM] Cl)中的反应在不到20(60)分钟内完全转化为HMF,选择性高达80%。甚至由于该IL的酸性特征,在[MIM] Cl溶剂中的反应不需要添加催化剂。在这项工作的第二步中,使用由PC-SAFT设计的非水两相反应系统NTPS对HMF进行原位提取。NTPS包含反应相([BMIM] Cl或[MIM] Cl)和萃取剂,即酮MEK,MIBK或乙酸乙酯之一。分析并评估了这些IL +有机溶剂NTPS的果糖转化率以及果糖和HMF的分配。PC-SAFT预测,在这项工作研究的所有系统中,对于果糖与HMF的反应以及从果糖中原位去除HMF而言,NTPS IL + MEK是最有前途的。实验结果可以验证PC-SAFT的预测,即IL + MEK NTPS可以将果糖有效地转化为HMF,并且HMF相对于果糖的分配选择性约为100%。由于[MIM] Cl的酸度,这种新的NTPS不需要额外的催化剂。它在20分钟时可实现87%的高反应选择性和93%的转化率,而且分离效率高。总共,











































 京公网安备 11010802027423号
京公网安备 11010802027423号