当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biological Basis for Threshold Responses to Methylating Agents.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.chemrestox.0c00052 Adam D Thomas 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.chemrestox.0c00052 Adam D Thomas 1
Affiliation
|
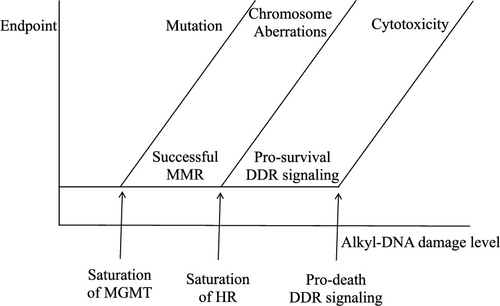
The cellular outcomes of chemical exposure are as much about the cellular response to the chemical as it is an effect of the chemical. We are growing in our understanding of the genotoxic interaction between chemistry and biology. For example, recent data has revealed the biological basis for mutation induction curves for a methylating chemical, which has been shown to be dependent on the repair capacity of the cells. However, this is just one end point in the toxicity pathway from chemical exposure to cell death. Much remains to be known in order for us to predict how cells will respond to a certain dose. Methylating agents, a subset of alkylating agents, are of particular interest, because of the variety of adverse genetic end points that can result, not only at increasing doses, but also over time. For instance, methylating agents are mutagenic, their potency, for this end point, is determined by the cellular repair capacity of an enzyme called methylguanine DNA-methyltransferase (MGMT) and its ability to repair the induceed methyl adducts. However, methyl adducts can become clastogenic. Erroneous biological processing will convert mutagenic adducts to clastogenic events in the form of double strand breaks (DSBs). How the cell responds to DSBs is via a cascade of protein kinases, which is called the DNA damage response (DDR), which will determine if the damage is repaired effectively, via homologous recombination, or with errors, via nonhomologous end joining, or whether the cell dies via apoptosis or enters senescence. The fate of cells may be determined by the extent of damage and the resulting strength of DDR signaling. Therefore, thresholds of damage may exist that determine cell fate. Such thresholds would be dependent on each of the repair and response mechanisms that these methyl adducts stimulate. The molecular mechanism of how methyl adducts kill cells is still to be fully resolved. If we are able to quantify each of these thresholds of damage for a given cell, then we can ascertain, of the many adducts that are induced, what proportion of them are mutagenic, what proportion are clastogenic, and how many of these clastogenic events are toxic. This review examines the possibility of dose and damage thresholds for methylating agents, from the perspective of the underlying evolutionary mechanisms that may be accountable.
中文翻译:

甲基化试剂阈值响应的生物学基础。
化学暴露的细胞结果与细胞对化学物质的反应一样多,因为它是一种效果的化学品。我们对化学和生物学之间的遗传毒性相互作用的理解不断加深。例如,最近的数据揭示了甲基化化学物质突变诱导曲线的生物学基础,这已被证明取决于细胞的修复能力。然而,这只是从化学暴露到细胞死亡的毒性途径的一个终点。为了预测细胞对特定剂量的反应,还有很多事情需要了解。甲基化剂是烷化剂的一个子集,特别令人感兴趣,因为不仅在剂量增加时,而且随着时间的推移,可能会导致各种不利的遗传终点。例如,甲基化剂具有致突变性,它们的效力,对于这个终点,由称为甲基鸟嘌呤 DNA 甲基转移酶 (MGMT) 的酶的细胞修复能力及其修复诱导的甲基加合物的能力决定。然而,甲基加合物会变成致裂体。错误的生物加工会将诱变加合物转化为双链断裂 (DSB) 形式的致裂事件。细胞对 DSB 的反应是通过一系列蛋白激酶,这被称为 DNA 损伤反应 (DDR),它将确定损伤是否得到有效修复,通过同源重组,或错误,通过非同源末端连接,或细胞通过细胞凋亡死亡或进入衰老。细胞的命运可能取决于损伤的程度和由此产生的 DDR 信号强度。因此,可能存在决定细胞命运的损伤阈值。这些阈值将取决于这些甲基加合物刺激的每种修复和反应机制。甲基加合物如何杀死细胞的分子机制仍有待完全解决。如果我们能够量化给定细胞的这些损伤阈值中的每一个,那么我们就可以确定在诱导的许多加合物中,它们中有多少是致突变的,有多少是致裂的,有多少是这些致裂事件是有毒的。本综述从可能负责的潜在进化机制的角度检查了甲基化剂的剂量和损伤阈值的可能性。如果我们能够量化给定细胞的这些损伤阈值中的每一个,那么我们就可以确定在诱导的许多加合物中,它们中有多少是致突变的,有多少是致裂的,有多少是这些致裂事件是有毒的。本综述从可能负责的潜在进化机制的角度检查了甲基化剂的剂量和损伤阈值的可能性。如果我们能够量化给定细胞的这些损伤阈值中的每一个,那么我们就可以确定在诱导的许多加合物中,它们中有多少是致突变的,有多少是致裂的,有多少是这些致裂事件是有毒的。本综述从可能负责的潜在进化机制的角度检查了甲基化剂的剂量和损伤阈值的可能性。
更新日期:2020-05-11
中文翻译:

甲基化试剂阈值响应的生物学基础。
化学暴露的细胞结果与细胞对化学物质的反应一样多,因为它是一种效果的化学品。我们对化学和生物学之间的遗传毒性相互作用的理解不断加深。例如,最近的数据揭示了甲基化化学物质突变诱导曲线的生物学基础,这已被证明取决于细胞的修复能力。然而,这只是从化学暴露到细胞死亡的毒性途径的一个终点。为了预测细胞对特定剂量的反应,还有很多事情需要了解。甲基化剂是烷化剂的一个子集,特别令人感兴趣,因为不仅在剂量增加时,而且随着时间的推移,可能会导致各种不利的遗传终点。例如,甲基化剂具有致突变性,它们的效力,对于这个终点,由称为甲基鸟嘌呤 DNA 甲基转移酶 (MGMT) 的酶的细胞修复能力及其修复诱导的甲基加合物的能力决定。然而,甲基加合物会变成致裂体。错误的生物加工会将诱变加合物转化为双链断裂 (DSB) 形式的致裂事件。细胞对 DSB 的反应是通过一系列蛋白激酶,这被称为 DNA 损伤反应 (DDR),它将确定损伤是否得到有效修复,通过同源重组,或错误,通过非同源末端连接,或细胞通过细胞凋亡死亡或进入衰老。细胞的命运可能取决于损伤的程度和由此产生的 DDR 信号强度。因此,可能存在决定细胞命运的损伤阈值。这些阈值将取决于这些甲基加合物刺激的每种修复和反应机制。甲基加合物如何杀死细胞的分子机制仍有待完全解决。如果我们能够量化给定细胞的这些损伤阈值中的每一个,那么我们就可以确定在诱导的许多加合物中,它们中有多少是致突变的,有多少是致裂的,有多少是这些致裂事件是有毒的。本综述从可能负责的潜在进化机制的角度检查了甲基化剂的剂量和损伤阈值的可能性。如果我们能够量化给定细胞的这些损伤阈值中的每一个,那么我们就可以确定在诱导的许多加合物中,它们中有多少是致突变的,有多少是致裂的,有多少是这些致裂事件是有毒的。本综述从可能负责的潜在进化机制的角度检查了甲基化剂的剂量和损伤阈值的可能性。如果我们能够量化给定细胞的这些损伤阈值中的每一个,那么我们就可以确定在诱导的许多加合物中,它们中有多少是致突变的,有多少是致裂的,有多少是这些致裂事件是有毒的。本综述从可能负责的潜在进化机制的角度检查了甲基化剂的剂量和损伤阈值的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号