Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphatidylinositol 3,5-bisphosphate regulates Ca2+ transport during yeast vacuolar fusion through the Ca2+ ATPase Pmc1.
Traffic ( IF 3.6 ) Pub Date : 2020-05-10 , DOI: 10.1111/tra.12736 Gregory E Miner 1 , Katherine D Sullivan 1 , Chi Zhang 1 , David Rivera-Kohr 1 , Annie Guo 1 , Logan R Hurst 1 , Ez C Ellis 1 , Matthew L Starr 1 , Brandon C Jones 1 , Rutilio A Fratti 1, 2
Traffic ( IF 3.6 ) Pub Date : 2020-05-10 , DOI: 10.1111/tra.12736 Gregory E Miner 1 , Katherine D Sullivan 1 , Chi Zhang 1 , David Rivera-Kohr 1 , Annie Guo 1 , Logan R Hurst 1 , Ez C Ellis 1 , Matthew L Starr 1 , Brandon C Jones 1 , Rutilio A Fratti 1, 2
Affiliation
|
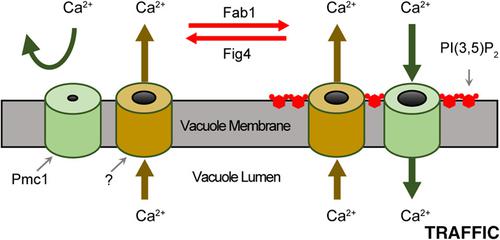
The transport of Ca2+ across membranes precedes the fusion and fission of various lipid bilayers. Yeast vacuoles under hyperosmotic stress become fragmented through fission events that requires the release of Ca2+ stores through the TRP channel Yvc1. This requires the phosphorylation of phosphatidylinositol‐3‐phosphate (PI3P) by the PI3P‐5‐kinase Fab1 to produce transient PI(3,5)P2 pools. Ca2+ is also released during vacuole fusion upon trans‐ SNARE complex assembly, however, its role remains unclear. The effect of PI(3,5)P2 on Ca2+ flux during fusion was independent of Yvc1. Here, we show that while low levels of PI(3,5)P2 were required for Ca2+ uptake into the vacuole, increased concentrations abolished Ca2+ efflux. This was as shown by the addition of exogenous dioctanoyl PI(3,5)P2 or increased endogenous production of by the hyperactive fab1 T2250A mutant. In contrast, the lack of PI(3,5)P2 on vacuoles from the kinase dead fab1 EEE mutant showed delayed and decreased Ca2+ uptake. The effects of PI(3,5)P2 were linked to the Ca2+ pump Pmc1, as its deletion rendered vacuoles resistant to the effects of excess PI(3,5)P2. Experiments with Verapamil inhibited Ca2+ uptake when added at the start of the assay, while adding it after Ca2+ had been taken up resulted in the rapid expulsion of Ca2+. Vacuoles lacking both Pmc1 and the H+/Ca2+ exchanger Vcx1 lacked the ability to take up Ca2+ and instead expelled it upon the addition of ATP. Together these data suggest that a balance of efflux and uptake compete during the fusion pathway and that the levels of PI(3,5)P2 can modulate which path predominates.
中文翻译:

磷脂酰肌醇 3,5-二磷酸通过 Ca2+ ATPase Pmc1 调节酵母液泡融合过程中的 Ca2+ 转运。
Ca 2+跨膜转运先于各种脂质双层的融合和裂变。高渗胁迫下的酵母液泡通过裂变事件破碎,裂变事件需要通过 TRP 通道 Yvc1释放 Ca 2+储存。这需要 PI3P-5-激酶 Fab1 磷酸化磷脂酰肌醇 3-磷酸 (PI3P) 以产生瞬时 PI(3,5)P 2池。Ca 2+在反式SNARE 复合体组装后的液泡融合过程中也会释放,但其作用尚不清楚。PI(3,5)P 2对融合过程中Ca 2+通量的影响与Yvc1 无关。在这里,我们表明虽然低水平的 PI(3,5)P 2Ca 2+吸收到液泡中是必需的,增加的浓度消除了Ca 2+流出。这通过添加外源性二辛酰基PI(3,5)P 2或由过度活跃的fab1 T2250A突变体增加的内源性产生来证明。相比之下,激酶死亡fab1 EEE突变体液泡上PI(3,5)P 2的缺乏显示出延迟和降低的 Ca 2+摄取。PI(3,5)P 2 的影响与Ca 2+泵Pmc1 相关,因为它的缺失使液泡对过量PI(3,5)P 2的影响具有抗性。维拉帕米抑制钙离子的实验2+在测定开始时被吸收,而在 Ca 2+被吸收后添加导致 Ca 2+的快速排出。缺乏 Pmc1 和 H + /Ca 2+交换器 Vcx1 的液泡缺乏吸收 Ca 2+的能力,而是在添加 ATP 时将其排出。这些数据一起表明,在融合途径期间外排和摄取的平衡竞争,并且 PI(3,5)P 2 的水平可以调节哪种途径占主导地位。
更新日期:2020-06-29
中文翻译:

磷脂酰肌醇 3,5-二磷酸通过 Ca2+ ATPase Pmc1 调节酵母液泡融合过程中的 Ca2+ 转运。
Ca 2+跨膜转运先于各种脂质双层的融合和裂变。高渗胁迫下的酵母液泡通过裂变事件破碎,裂变事件需要通过 TRP 通道 Yvc1释放 Ca 2+储存。这需要 PI3P-5-激酶 Fab1 磷酸化磷脂酰肌醇 3-磷酸 (PI3P) 以产生瞬时 PI(3,5)P 2池。Ca 2+在反式SNARE 复合体组装后的液泡融合过程中也会释放,但其作用尚不清楚。PI(3,5)P 2对融合过程中Ca 2+通量的影响与Yvc1 无关。在这里,我们表明虽然低水平的 PI(3,5)P 2Ca 2+吸收到液泡中是必需的,增加的浓度消除了Ca 2+流出。这通过添加外源性二辛酰基PI(3,5)P 2或由过度活跃的fab1 T2250A突变体增加的内源性产生来证明。相比之下,激酶死亡fab1 EEE突变体液泡上PI(3,5)P 2的缺乏显示出延迟和降低的 Ca 2+摄取。PI(3,5)P 2 的影响与Ca 2+泵Pmc1 相关,因为它的缺失使液泡对过量PI(3,5)P 2的影响具有抗性。维拉帕米抑制钙离子的实验2+在测定开始时被吸收,而在 Ca 2+被吸收后添加导致 Ca 2+的快速排出。缺乏 Pmc1 和 H + /Ca 2+交换器 Vcx1 的液泡缺乏吸收 Ca 2+的能力,而是在添加 ATP 时将其排出。这些数据一起表明,在融合途径期间外排和摄取的平衡竞争,并且 PI(3,5)P 2 的水平可以调节哪种途径占主导地位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号