当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cardiac troponin and tropomyosin bind to F-actin cooperatively, as revealed by fluorescence microscopy.
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-06-18 , DOI: 10.1002/2211-5463.12876 Christopher Solís 1 , John M Robinson 2
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-06-18 , DOI: 10.1002/2211-5463.12876 Christopher Solís 1 , John M Robinson 2
Affiliation
|
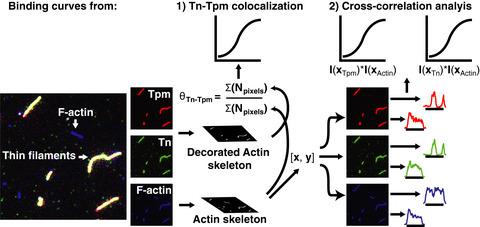
In cardiac muscle, binding of troponin (Tn) and tropomyosin (Tpm) to filamentous (F)‐actin forms thin filaments capable of Ca2+‐dependent regulation of contraction. Tpm binds to F‐actin in a head‐to‐tail fashion, while Tn stabilizes these linkages. Valuable structural and functional information has come from biochemical, X‐ray, and electron microscopy data. However, the use of fluorescence microscopy to study thin filament assembly remains relatively underdeveloped. Here, triple fluorescent labeling of Tn, Tpm, and F‐actin allowed us to track thin filament assembly by fluorescence microscopy. It is shown here that Tn and Tpm molecules self‐organize on actin filaments and give rise to decorated and undecorated regions. Binding curves based on colocalization of Tn and Tpm on F‐actin exhibit cooperative binding with a dissociation constant Kd of ~ 0.5 µm that is independent of the Ca2+ concentration. Binding isotherms based on the intensity profile of fluorescently labeled Tn and Tpm on F‐actin show that binding of Tn is less cooperative relative to Tpm. Computational modeling of Tn‐Tpm binding to F‐actin suggests two equilibrium steps involving the binding of an initial Tn‐Tpm unit (nucleation) and subsequent recruitment of adjacent Tn‐Tpm units (elongation) that stabilize the assembly. The results presented here highlight the utility of employing fluorescence microscopy to study supramolecular protein assemblies.
中文翻译:

如荧光显微镜所示,心脏肌钙蛋白和原肌球蛋白协同结合 F-肌动蛋白。
在心肌中,肌钙蛋白 (Tn) 和原肌球蛋白 (Tpm) 与丝状 (F)-肌动蛋白结合形成能够吸收 Ca 2+ 的细丝收缩的依赖性调节。Tpm 以头对尾的方式与 F-肌动蛋白结合,而 Tn 稳定这些联系。有价值的结构和功能信息来自生化、X 射线和电子显微镜数据。然而,使用荧光显微镜研究细丝组装仍然相对不发达。在这里,Tn、Tpm 和 F-肌动蛋白的三重荧光标记使我们能够通过荧光显微镜跟踪细丝组装。这里显示 Tn 和 Tpm 分子在肌动蛋白丝上自组织并产生装饰和未装饰区域。上结合根据Tn的和TPM的共定位曲线F-肌动蛋白表现出合作的解离常数结合ķ d的0.5〜μ米独立于的Ca 2+专注。基于荧光标记的 Tn 和 Tpm 在 F-肌动蛋白上的强度分布的结合等温线表明,相对于 Tpm,Tn 的结合协作性较差。Tn-Tpm 与 F-肌动蛋白结合的计算模型表明两个平衡步骤,包括初始 Tn-Tpm 单元的结合(成核)和随后稳定组装的相邻 Tn-Tpm 单元的募集(伸长)。这里介绍的结果突出了使用荧光显微镜研究超分子蛋白质组装的实用性。
更新日期:2020-06-18
中文翻译:

如荧光显微镜所示,心脏肌钙蛋白和原肌球蛋白协同结合 F-肌动蛋白。
在心肌中,肌钙蛋白 (Tn) 和原肌球蛋白 (Tpm) 与丝状 (F)-肌动蛋白结合形成能够吸收 Ca 2+ 的细丝收缩的依赖性调节。Tpm 以头对尾的方式与 F-肌动蛋白结合,而 Tn 稳定这些联系。有价值的结构和功能信息来自生化、X 射线和电子显微镜数据。然而,使用荧光显微镜研究细丝组装仍然相对不发达。在这里,Tn、Tpm 和 F-肌动蛋白的三重荧光标记使我们能够通过荧光显微镜跟踪细丝组装。这里显示 Tn 和 Tpm 分子在肌动蛋白丝上自组织并产生装饰和未装饰区域。上结合根据Tn的和TPM的共定位曲线F-肌动蛋白表现出合作的解离常数结合ķ d的0.5〜μ米独立于的Ca 2+专注。基于荧光标记的 Tn 和 Tpm 在 F-肌动蛋白上的强度分布的结合等温线表明,相对于 Tpm,Tn 的结合协作性较差。Tn-Tpm 与 F-肌动蛋白结合的计算模型表明两个平衡步骤,包括初始 Tn-Tpm 单元的结合(成核)和随后稳定组装的相邻 Tn-Tpm 单元的募集(伸长)。这里介绍的结果突出了使用荧光显微镜研究超分子蛋白质组装的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号