当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Histone H4 variant, H4G, drives ribosomal RNA transcription and breast cancer cell proliferation by loosening nucleolar chromatin structure.
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-05-09 , DOI: 10.1002/jcp.29770 Matthew Y H Pang 1 , Xulun Sun 1 , Juan Ausió 2 , Toyotaka Ishibashi 1
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-05-09 , DOI: 10.1002/jcp.29770 Matthew Y H Pang 1 , Xulun Sun 1 , Juan Ausió 2 , Toyotaka Ishibashi 1
Affiliation
|
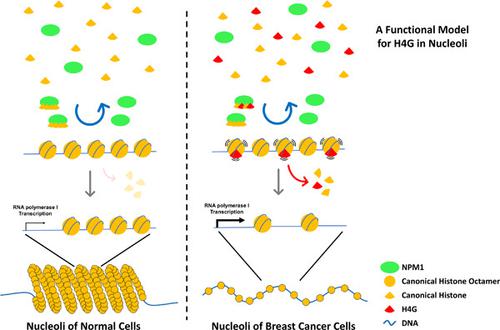
The hominidae‐specific histone variant H4G is expressed in breast cancer patients in a stage‐dependent manner. H4G localizes primarily in the nucleoli via its interaction with nucleophosmin (NPM1). H4G is involved in rDNA transcription and ribosome biogenesis, which facilitates breast cancer cell proliferation. However, the molecular mechanism underlying this process remains unknown. Here, we show that H4G is not stably incorporated into nucleolar chromatin, even with the chaperoning assistance of NPM1. H4G likely form transient nucleosome‐like‐structure that undergoes rapid dissociation. In addition, the nucleolar chromatin in H4GKO cells is more compact than WT cells. Altogether, our results suggest that H4G relaxes the nucleolar chromatin and enhances rRNA transcription by forming destabilized nucleosome in breast cancer cells.
中文翻译:

H4G组蛋白H4变体通过松散核仁染色质结构来驱动核糖体RNA转录和乳腺癌细胞增殖。
人科特定的组蛋白变体H4G在乳腺癌患者中以阶段依赖的方式表达。H4G主要通过与核磷脂(NPM1)的相互作用而定位在核仁中。H4G参与rDNA转录和核糖体生物发生,从而促进乳腺癌细胞增殖。但是,这一过程的分子机制仍然未知。在这里,我们显示即使在NPM1的陪伴下,H4G也不能稳定地整合到核仁染色质中。H4G可能形成经历快速解离的瞬时核小体样结构。此外,H4GKO细胞中的核仁染色质比WT细胞致密。总之,我们的结果表明H4G通过在乳腺癌细胞中形成不稳定的核小体来放松核仁染色质并增强rRNA转录。
更新日期:2020-05-09
中文翻译:

H4G组蛋白H4变体通过松散核仁染色质结构来驱动核糖体RNA转录和乳腺癌细胞增殖。
人科特定的组蛋白变体H4G在乳腺癌患者中以阶段依赖的方式表达。H4G主要通过与核磷脂(NPM1)的相互作用而定位在核仁中。H4G参与rDNA转录和核糖体生物发生,从而促进乳腺癌细胞增殖。但是,这一过程的分子机制仍然未知。在这里,我们显示即使在NPM1的陪伴下,H4G也不能稳定地整合到核仁染色质中。H4G可能形成经历快速解离的瞬时核小体样结构。此外,H4GKO细胞中的核仁染色质比WT细胞致密。总之,我们的结果表明H4G通过在乳腺癌细胞中形成不稳定的核小体来放松核仁染色质并增强rRNA转录。



























 京公网安备 11010802027423号
京公网安备 11010802027423号