当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Consequences of the 1,2,3-Triazole as an Amide Bioisostere in Analogues of the Cystic Fibrosis Drugs VX-809 and VX-770.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-08 , DOI: 10.1002/cmdc.202000220 Jake E Doiron 1 , Christina A Le 2 , John Bacsa 3 , Gary W Breton 1 , Kenneth L Martin 1 , Stephen G Aller 2 , Mark Turlington 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-08 , DOI: 10.1002/cmdc.202000220 Jake E Doiron 1 , Christina A Le 2 , John Bacsa 3 , Gary W Breton 1 , Kenneth L Martin 1 , Stephen G Aller 2 , Mark Turlington 1
Affiliation
|
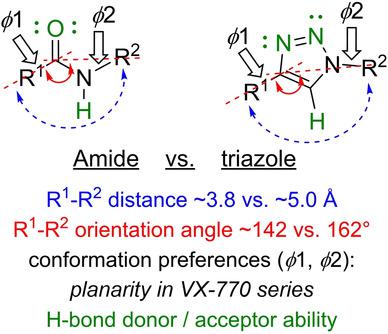
Although the 1,2,3‐triazole is a commonly used amide bioisostere in medicinal chemistry, the structural implications of this replacement have not been fully studied. Employing X‐ray crystallography and computational studies, we report the spatial and electronic consequences of replacing an amide with the triazole in analogues of cystic fibrosis drugs in the VX‐770 and VX‐809 series. Crystallographic analyses quantify subtle differences in the relative positions and conformational preferences of the R1 and R2 substituents attached to the amide and triazole bioisosteres. Computational studies derived from the X‐ray data highlight the improved hydrogen bonding donor and acceptor capabilities of the amide in comparison to the triazole. This analysis of the spatial and electronic differences between the amide and 1,2,3‐triazole will inform medicinal chemists as they consider using the triazole as an amide bioisostere.
中文翻译:

1,2,3-三唑作为囊性纤维化药物 VX-809 和 VX-770 类似物中的酰胺生物等排体的结构后果。
尽管 1,2,3-三唑是药物化学中常用的酰胺生物电子等排体,但尚未充分研究这种替代物的结构含义。利用 X 射线晶体学和计算研究,我们报告了在 VX-770 和 VX-809 系列囊性纤维化药物类似物中用三唑代替酰胺的空间和电子后果。晶体学分析量化了 R 1和 R 2的相对位置和构象偏好的细微差异连接到酰胺和三唑生物电子等排体的取代基。来自 X 射线数据的计算研究强调了与三唑相比,酰胺具有改进的氢键供体和受体能力。对酰胺和 1,2,3-三唑之间空间和电子差异的分析将为药物化学家提供信息,因为他们考虑使用三唑作为酰胺生物等排体。
更新日期:2020-05-08
中文翻译:

1,2,3-三唑作为囊性纤维化药物 VX-809 和 VX-770 类似物中的酰胺生物等排体的结构后果。
尽管 1,2,3-三唑是药物化学中常用的酰胺生物电子等排体,但尚未充分研究这种替代物的结构含义。利用 X 射线晶体学和计算研究,我们报告了在 VX-770 和 VX-809 系列囊性纤维化药物类似物中用三唑代替酰胺的空间和电子后果。晶体学分析量化了 R 1和 R 2的相对位置和构象偏好的细微差异连接到酰胺和三唑生物电子等排体的取代基。来自 X 射线数据的计算研究强调了与三唑相比,酰胺具有改进的氢键供体和受体能力。对酰胺和 1,2,3-三唑之间空间和电子差异的分析将为药物化学家提供信息,因为他们考虑使用三唑作为酰胺生物等排体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号