当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
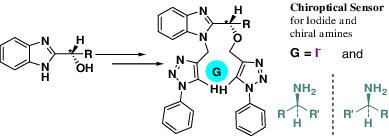
Chiral benzimidazole derived bis‐phenyl triazoles as chiroptical sensors for iodide and chiral amines
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-05-08 , DOI: 10.1002/jhet.3993 Marina E. John 1 , Anil V. Karnik 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-05-08 , DOI: 10.1002/jhet.3993 Marina E. John 1 , Anil V. Karnik 1
Affiliation
|
A series of chiral 2‐hydroxy ethyl/benzyl benzimidazole based aryl triazole tweezers have been prepared using click chemistry in high yields. Chiral pool strategy has been used to obtain the benzimidazole‐based tweezers in very high enantiomerically enriched form. The aryl triazole tweezers, S ‐(−)‐5a and S ‐(+)‐8a displayed a high degree of selectivity for iodide anion over other anions, including other halides. The aryl triazole tweezers, S ‐(−)‐5a and S ‐(+ )‐8a display significant enantio‐discrimination for chiral amines. The chiral recognition studies were carried out using UV and circular dichroism (CD) spectroscopy. NMR analysis has been used for establishing the sites for ligation of the iodide anion.
中文翻译:

手性苯并咪唑衍生的双苯基三唑作为碘化物和手性胺的手性传感器
使用点击化学,已经以高收率制备了一系列手性的2-羟乙基/苄基苯并咪唑基芳基三唑镊子。手性池策略已被用于以对映体富集的形式获得基于苯并咪唑的镊子。芳基三唑镊子S -(-)- 5a和S -(+)- 8a对碘离子的选择性比其他阴离子(包括其他卤化物)高。芳基三唑镊子S -(-)- 5a和S -(+)- 8a对手性胺显示出显着的对映异构性。使用紫外线和圆二色性(CD)光谱进行手性识别研究。NMR分析已用于建立碘阴离子的连接位点。
更新日期:2020-07-15
中文翻译:

手性苯并咪唑衍生的双苯基三唑作为碘化物和手性胺的手性传感器
使用点击化学,已经以高收率制备了一系列手性的2-羟乙基/苄基苯并咪唑基芳基三唑镊子。手性池策略已被用于以对映体富集的形式获得基于苯并咪唑的镊子。芳基三唑镊子S -(-)- 5a和S -(+)- 8a对碘离子的选择性比其他阴离子(包括其他卤化物)高。芳基三唑镊子S -(-)- 5a和S -(+)- 8a对手性胺显示出显着的对映异构性。使用紫外线和圆二色性(CD)光谱进行手性识别研究。NMR分析已用于建立碘阴离子的连接位点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号