Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2020-05-07 , DOI: 10.1016/j.jwpe.2020.101284 Tansir Ahamad , Mu Naushad , Saad M. Alshehri
|
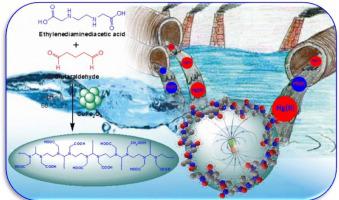
Discharge of heavy metals without proper treatment of the industrial wastewater and resulted damages to the aquatic ecosystems and human health. In the present study, a magnetic adsorbent containing carboxylic acid and amino functional groups was fabricated using ethylenediaminediacetic (EDDA) acid, glutaraldehyde and CuFe2O4 nanoparticles. The structure and composition of the fabricated nanocomposite (EG@ CuFe2O4) was analyzed by FTIR, TGA, XRD, BET, SEM, TEM, XPS etc, and employ for the removal of Hg(II) and Pb(II) from aqueous medium. The adsorption kinetics and adsorption isotherm model was used for further characterization of the adsorption process for the metal ions, and support the pseudo- second order kinetics and Langmuir model. The thermodynamic parameters (ΔGo < 0, ΔHo > 0) support, spontaneous and endothermic adsorption. All these results revealed that the as fabricated magnetic EG@ CuFe2O4 nanocomposite can be used as an eco-friendly, proficient and cheap adsorbent for the adsorption of heavy metals form aqueous solution.
中文翻译:

磁性聚合树脂的制造,用于从水性介质中去除有毒金属:动力学和吸附机理
不经适当处理工业废水就排放重金属,对水生生态系统和人类健康造成损害。在本研究中,使用乙二胺二乙酸(EDDA)酸,戊二醛和CuFe 2 O 4纳米颗粒制备了包含羧酸和氨基官能团的磁性吸附剂。纳米复合材料(EG @ CuFe 2 O 4的结构和组成)用FTIR,TGA,XRD,BET,SEM,TEM,XPS等进行分析,并用于从水性介质中去除Hg(II)和Pb(II)。吸附动力学和吸附等温线模型用于进一步表征金属离子的吸附过程,并支持拟二级动力学和Langmuir模型。热力学参数(ΔG Ø <0,ΔH ö > 0)的支持,自发的吸热吸附。所有这些结果表明,所制备的磁性EG @ CuFe 2 O 4纳米复合材料可以用作生态友好,熟练且廉价的吸附剂,用于从水溶液中吸附重金属。











































 京公网安备 11010802027423号
京公网安备 11010802027423号