当前位置:
X-MOL 学术
›
Hum. Mutat.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions.
Human Mutation ( IF 3.3 ) Pub Date : 2020-05-07 , DOI: 10.1002/humu.24033 Marie Balzotti 1 , Linyan Meng 2 , Dale Muzzey 1 , Katherine Johansen Taber 1 , Kyle Beauchamp 1, 3 , Myriad Genetics Curation Team 1 , Baylor Genetics Curation Team 2 , Rebecca Mar-Heyming 1, 4 , Bethany Buckley 1, 5 , Krista Moyer 1
Human Mutation ( IF 3.3 ) Pub Date : 2020-05-07 , DOI: 10.1002/humu.24033 Marie Balzotti 1 , Linyan Meng 2 , Dale Muzzey 1 , Katherine Johansen Taber 1 , Kyle Beauchamp 1, 3 , Myriad Genetics Curation Team 1 , Baylor Genetics Curation Team 2 , Rebecca Mar-Heyming 1, 4 , Bethany Buckley 1, 5 , Krista Moyer 1
Affiliation
|
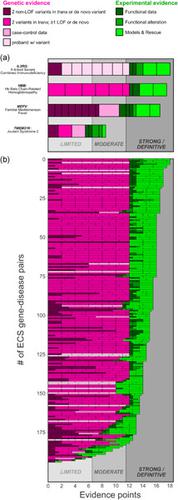
Clinical guidelines consider expanded carrier screening (ECS) to be an acceptable method of carrier screening. However, broader guideline support and payer adoption require evidence for associations between the genes on ECS panels and the conditions for which they aim to identify carriers. We applied a standardized framework for evaluation of gene‐disease association to assess the clinical validity of conditions screened by ECS panels. The Clinical Genome Resource (ClinGen) gene curation framework was used to assess genetic and experimental evidence of associations between 208 genes and conditions screened on two commercial ECS panels. Twenty‐one conditions were previously classified by ClinGen, and the remaining 187 were evaluated by curation teams at two laboratories. To ensure consistent application of the framework across the laboratories, concordance was evaluated on a subset of conditions. All 208 evaluated conditions met the evidence threshold for supporting a gene‐disease association. Furthermore, 203 of 208 (98%) achieved the strongest (“Definitive”) level of gene‐disease association. All conditions evaluated by both commercial laboratories were similarly classified. Assessment using the ClinGen standardized framework revealed strong evidence of gene‐disease association for conditions on two ECS panels. This result establishes the disease‐level clinical validity of the panels considered herein.
中文翻译:

扩大携带者筛查的临床有效性:评估 200 多种条件下的基因-疾病关系。
临床指南认为扩大携带者筛查 (ECS) 是可接受的携带者筛查方法。然而,更广泛的指南支持和付款人的采用需要有证据证明 ECS 面板上的基因与其旨在识别携带者的条件之间存在关联。我们应用了评估基因-疾病关联的标准化框架来评估 ECS 小组筛选条件的临床有效性。临床基因组资源 (ClinGen) 基因管理框架用于评估在两个商业 ECS 面板上筛选的 208 个基因和条件之间关联的遗传和实验证据。ClinGen 之前对 21 种情况进行了分类,其余 187 种情况由两个实验室的管理团队进行评估。为确保该框架在实验室中的一致应用,在条件子集上评估一致性。所有 208 项评估条件均符合支持基因-疾病关联的证据阈值。此外,208 人中有 203 人(98%)达到了最强(“确定”)水平的基因-疾病关联。两个商业实验室评估的所有条件都进行了类似的分类。使用 ClinGen 标准化框架的评估揭示了两个 ECS 面板上基因与疾病关联的有力证据。该结果确立了本文考虑的小组的疾病水平临床有效性。两个商业实验室评估的所有条件都进行了类似的分类。使用 ClinGen 标准化框架的评估揭示了两个 ECS 面板上基因与疾病关联的有力证据。该结果确立了本文考虑的小组的疾病水平临床有效性。两个商业实验室评估的所有条件都进行了类似的分类。使用 ClinGen 标准化框架的评估揭示了两个 ECS 面板上基因与疾病关联的有力证据。该结果确立了本文考虑的小组的疾病水平临床有效性。
更新日期:2020-05-07
中文翻译:

扩大携带者筛查的临床有效性:评估 200 多种条件下的基因-疾病关系。
临床指南认为扩大携带者筛查 (ECS) 是可接受的携带者筛查方法。然而,更广泛的指南支持和付款人的采用需要有证据证明 ECS 面板上的基因与其旨在识别携带者的条件之间存在关联。我们应用了评估基因-疾病关联的标准化框架来评估 ECS 小组筛选条件的临床有效性。临床基因组资源 (ClinGen) 基因管理框架用于评估在两个商业 ECS 面板上筛选的 208 个基因和条件之间关联的遗传和实验证据。ClinGen 之前对 21 种情况进行了分类,其余 187 种情况由两个实验室的管理团队进行评估。为确保该框架在实验室中的一致应用,在条件子集上评估一致性。所有 208 项评估条件均符合支持基因-疾病关联的证据阈值。此外,208 人中有 203 人(98%)达到了最强(“确定”)水平的基因-疾病关联。两个商业实验室评估的所有条件都进行了类似的分类。使用 ClinGen 标准化框架的评估揭示了两个 ECS 面板上基因与疾病关联的有力证据。该结果确立了本文考虑的小组的疾病水平临床有效性。两个商业实验室评估的所有条件都进行了类似的分类。使用 ClinGen 标准化框架的评估揭示了两个 ECS 面板上基因与疾病关联的有力证据。该结果确立了本文考虑的小组的疾病水平临床有效性。两个商业实验室评估的所有条件都进行了类似的分类。使用 ClinGen 标准化框架的评估揭示了两个 ECS 面板上基因与疾病关联的有力证据。该结果确立了本文考虑的小组的疾病水平临床有效性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号