当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High‐Voltage Oxygen‐Redox‐Based Cathode for Rechargeable Sodium‐Ion Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-05-07 , DOI: 10.1002/aenm.202001111 Aishuak Konarov 1, 2 , Hee Jae Kim 1 , Jae‐Hyeon Jo 1 , Natalia Voronina 1 , Yongseok Lee 1 , Zhumabay Bakenov 2 , Jongsoon Kim 1 , Seung‐Taek Myung 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-05-07 , DOI: 10.1002/aenm.202001111 Aishuak Konarov 1, 2 , Hee Jae Kim 1 , Jae‐Hyeon Jo 1 , Natalia Voronina 1 , Yongseok Lee 1 , Zhumabay Bakenov 2 , Jongsoon Kim 1 , Seung‐Taek Myung 1
Affiliation
|
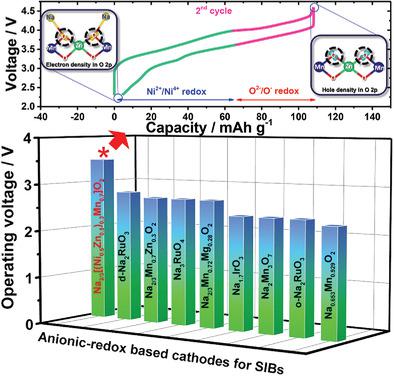
Recently, anionic‐redox‐based materials have shown promising electrochemical performance as cathode materials for sodium‐ion batteries. However, one of the limiting factors in the development of oxygen‐redox‐based electrodes is their low operating voltage. In this study, the operating voltage of oxygen‐redox‐based electrodes is raised by incorporating nickel into P2‐type Na2/3[Zn0.3Mn0.7]O2 in such a way that the zinc is partially substituted by nickel. As designed, the resulting P2‐type Na2/3[(Ni0.5Zn0.5)0.3Mn0.7]O2 electrode exhibits an average operating voltage of 3.5 V and retains 95% of its initial capacity after 200 cycles in the voltage range of 2.3–4.6 V at 0.1C (26 mA g−1). Operando X‐ray diffraction analysis reveals the reversible phase transition: P2 to OP4 phase on charge and recovery to the P2 phase on discharge. Moreover, ex situ X‐ray absorption near edge structure and X‐ray photoelectron spectroscopy studies reveal that the capacity is generated by the combination of Ni2+/Ni4+ and O2−/O1− redox pairs, which is supported by first‐principles calculations. It is thought that this kind of high voltage redox species combined with oxygen redox could be an interesting approach to further increase energy density of cathode materials for not only sodium‐based rechargeable batteries, but other alkali‐ion battery systems.
中文翻译:

用于可充电钠离子电池的高压氧-氧化还原基阴极
最近,基于阴离子-氧化还原的材料已显示出有希望的电化学性能,作为钠离子电池的阴极材料。然而,基于氧-氧化还原的电极发展的限制因素之一是它们的低工作电压。在这项研究中,通过将镍掺入P2型Na 2/3 [Zn 0.3 Mn 0.7 ] O 2中来提高氧-氧化还原基电极的工作电压,使得锌被镍部分取代。按照设计,生成的P2型Na 2/3 [(Ni 0.5 Zn 0.5)0.3 Mn 0.7 ] O 2电极的平均工作电压为3.5 V,在0.1C(26 mA g -1)的2.3–4.6 V电压范围内,经过200个循环后,仍能保持其初始容量的95%。Operando X射线衍射分析揭示了可逆的相变:充电时P2到OP4相,放电时恢复到P2相。此外,边缘结构的异位X射线吸收和X射线光电子能谱研究表明,容量是由Ni 2+ / Ni 4+和O 2- / O 1-的组合产生的氧化还原对,这是第一性原理计算所支持的。人们认为,这种高电压氧化还原物质与氧气氧化还原物质的结合可能是一种有趣的方法,可以进一步提高不仅用于钠基可再充电电池,而且用于其他碱离子电池系统的阴极材料的能量密度。
更新日期:2020-06-23
中文翻译:

用于可充电钠离子电池的高压氧-氧化还原基阴极
最近,基于阴离子-氧化还原的材料已显示出有希望的电化学性能,作为钠离子电池的阴极材料。然而,基于氧-氧化还原的电极发展的限制因素之一是它们的低工作电压。在这项研究中,通过将镍掺入P2型Na 2/3 [Zn 0.3 Mn 0.7 ] O 2中来提高氧-氧化还原基电极的工作电压,使得锌被镍部分取代。按照设计,生成的P2型Na 2/3 [(Ni 0.5 Zn 0.5)0.3 Mn 0.7 ] O 2电极的平均工作电压为3.5 V,在0.1C(26 mA g -1)的2.3–4.6 V电压范围内,经过200个循环后,仍能保持其初始容量的95%。Operando X射线衍射分析揭示了可逆的相变:充电时P2到OP4相,放电时恢复到P2相。此外,边缘结构的异位X射线吸收和X射线光电子能谱研究表明,容量是由Ni 2+ / Ni 4+和O 2- / O 1-的组合产生的氧化还原对,这是第一性原理计算所支持的。人们认为,这种高电压氧化还原物质与氧气氧化还原物质的结合可能是一种有趣的方法,可以进一步提高不仅用于钠基可再充电电池,而且用于其他碱离子电池系统的阴极材料的能量密度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号