当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promoting the sulfur conversion kinetics via a solid auxiliary redox couple embedded in the cathode of Li–S batteries
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-05-08 , DOI: 10.1039/d0se00553c Girum Girma Bizuneh 1, 2, 3, 4, 5 , Jingmin Fan 1, 2, 3, 4, 5 , Pan Xu 1, 2, 3, 4, 5 , Ruming Yuan 1, 2, 3, 4, 5 , Lin Cao 6, 7, 8 , Mingsen Zheng 1, 2, 3, 4, 5 , Quan-Feng Dong 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-05-08 , DOI: 10.1039/d0se00553c Girum Girma Bizuneh 1, 2, 3, 4, 5 , Jingmin Fan 1, 2, 3, 4, 5 , Pan Xu 1, 2, 3, 4, 5 , Ruming Yuan 1, 2, 3, 4, 5 , Lin Cao 6, 7, 8 , Mingsen Zheng 1, 2, 3, 4, 5 , Quan-Feng Dong 1, 2, 3, 4, 5
Affiliation
|
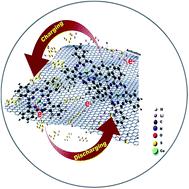
The sluggish kinetics of the sulfur conversion reactions in Li–S batteries is a critical challenge for their application. Thus, many studies have been dedicated to promoting the conversion process by including either functionalized matrix materials in the cathode or redox mediators in the electrolyte. Herein, by embedding a solid auxiliary redox species, we designed and prepared a composite that can be used as a matrix for a sulfur cathode. Differing from the conventional method, in which all the so-called redox mediators utilized are dissolved in the electrolyte, we employed a solid auxiliary redox species, a cobalt phthalocyanine complex supported on graphene structures, as skeleton materials of sulfur to promote the conversion kinetics in Li–S batteries. The graphene–cobalt phthalocyanine hybrid played a prominent role in promoting the kinetics of polysulfide conversion with multiple functions of reducing the activation energy hill, transferring electrons, suppressing the shuttle effect, etc. Consequently, the Li–S battery with the graphene–cobalt phthalocyanine–sulfur cathode showed better electrochemical performance than the battery with the graphene–sulfur cathode. Typically, a high initial capacity of 1400 mA h g−1 was achieved at 0.1C during the initial activation cycle, and the 1st and 300th cycle capacities at 0.5C were 1116 and 860 mA h g−1, respectively. Additionally, at a rate of 0.3C, high capacities of 1182 and 869 mA h g−1 were obtained during the 1st and 500th cycles, respectively. Furthermore, enhanced rate capability was achieved, delivering a capacity of 874 mA h g−1 at a rate of 2C.
中文翻译:

通过嵌入Li–S电池阴极的固体辅助氧化还原对促进硫的转化动力学
Li-S电池中硫转化反应的缓慢动力学是其应用中的关键挑战。因此,许多研究致力于通过在阴极中包括功能化基质材料或在电解质中包括氧化还原介体来促进转化过程。本文中,通过嵌入固体辅助氧化还原物质,我们设计并制备了可用作硫阴极基质的复合材料。与常规方法不同,在常规方法中,所有利用的所谓氧化还原介体都溶解在电解质中,我们采用了固态辅助氧化还原物质(负载在石墨烯结构上的钴酞菁配合物)作为硫的骨架材料,以促进硫的转化动力学。锂电池。等因此,锂硫电池与石墨烯-钴酞菁-硫阴极表现出比电池与石墨烯-硫阴极较好的电化学性能。典型地,1400毫安Hg的高初始容量-1是在0.1C的初始激活周期期间实现,和1日和300倍循环的容量以0.5C分别为1116和860毫安汞柱-1,分别。另外,在0.3C的速率,以及1182869毫安汞的高容量-1中的1期间获得的第一和500个周期分别。此外,实现了增强的速率能力,以2C的速率提供了874 mA hg -1的容量。
更新日期:2020-06-30
中文翻译:

通过嵌入Li–S电池阴极的固体辅助氧化还原对促进硫的转化动力学
Li-S电池中硫转化反应的缓慢动力学是其应用中的关键挑战。因此,许多研究致力于通过在阴极中包括功能化基质材料或在电解质中包括氧化还原介体来促进转化过程。本文中,通过嵌入固体辅助氧化还原物质,我们设计并制备了可用作硫阴极基质的复合材料。与常规方法不同,在常规方法中,所有利用的所谓氧化还原介体都溶解在电解质中,我们采用了固态辅助氧化还原物质(负载在石墨烯结构上的钴酞菁配合物)作为硫的骨架材料,以促进硫的转化动力学。锂电池。等因此,锂硫电池与石墨烯-钴酞菁-硫阴极表现出比电池与石墨烯-硫阴极较好的电化学性能。典型地,1400毫安Hg的高初始容量-1是在0.1C的初始激活周期期间实现,和1日和300倍循环的容量以0.5C分别为1116和860毫安汞柱-1,分别。另外,在0.3C的速率,以及1182869毫安汞的高容量-1中的1期间获得的第一和500个周期分别。此外,实现了增强的速率能力,以2C的速率提供了874 mA hg -1的容量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号