当前位置:
X-MOL 学术
›
J. Theor. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homo-Oligomerisation in Signal Transduction: Dynamics, Homeostasis, Ultrasensitivity, Bistability.
Journal of Theoretical Biology ( IF 1.9 ) Pub Date : 2020-05-08 , DOI: 10.1016/j.jtbi.2020.110305 Daniel Koch 1
Journal of Theoretical Biology ( IF 1.9 ) Pub Date : 2020-05-08 , DOI: 10.1016/j.jtbi.2020.110305 Daniel Koch 1
Affiliation
|
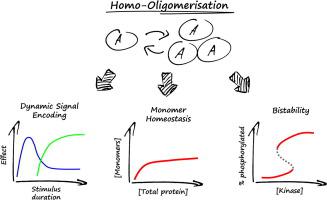
Homo-oligomerisation of proteins is a ubiquitous phenomenon whose exact role remains unclear in many cases. To identify novel functions, this paper provides an exploration of general dynamical mathematical models of homo-oligomerisation. Simulation and analysis of these models show that homo-oligomerisation on its own allows for a remarkable variety of complex dynamic and steady state regulatory behaviour such as transient overshoots or homeostatic control of monomer concentration. If post-translational modifications are considered, however, conventional mass action kinetics lead to thermodynamic inconsistencies due to asymmetric combinatorial expansion of reaction routes. Introducing a conservation principle to balance rate equations re-establishes thermodynamic consistency. Using such balanced models it is shown that oligomerisation can lead to bistability by enabling pseudo-multisite modification and kinetic pseudo-cooperativity via multi-enzyme regulation, thereby constituting a novel motif for bistable modification reactions. Due to these potential signal processing capabilities, homo-oligomerisation could play far more versatile roles in signal transduction than previously appreciated.
中文翻译:

信号转导中的同源寡聚化:动力学、稳态、超敏性、双稳定性。
蛋白质的同源寡聚化是一种普遍存在的现象,其确切作用在许多情况下仍不清楚。为了识别新功能,本文对同源齐聚的一般动态数学模型进行了探索。这些模型的模拟和分析表明,同源寡聚化本身可以实现多种复杂的动态和稳态调节行为,例如单体浓度的瞬时超调或稳态控制。然而,如果考虑翻译后修饰,由于反应路线的不对称组合扩展,传统的质量作用动力学会导致热力学不一致。引入守恒原理来平衡速率方程重新建立热力学一致性。使用这种平衡模型表明,寡聚可以通过多酶调节实现伪多位点修饰和动力学伪协同性,从而导致双稳态,从而构成双稳态修饰反应的新基序。由于这些潜在的信号处理能力,同源寡聚化在信号转导中发挥的作用比以前想象的要广泛得多。
更新日期:2020-05-08
中文翻译:

信号转导中的同源寡聚化:动力学、稳态、超敏性、双稳定性。
蛋白质的同源寡聚化是一种普遍存在的现象,其确切作用在许多情况下仍不清楚。为了识别新功能,本文对同源齐聚的一般动态数学模型进行了探索。这些模型的模拟和分析表明,同源寡聚化本身可以实现多种复杂的动态和稳态调节行为,例如单体浓度的瞬时超调或稳态控制。然而,如果考虑翻译后修饰,由于反应路线的不对称组合扩展,传统的质量作用动力学会导致热力学不一致。引入守恒原理来平衡速率方程重新建立热力学一致性。使用这种平衡模型表明,寡聚可以通过多酶调节实现伪多位点修饰和动力学伪协同性,从而导致双稳态,从而构成双稳态修饰反应的新基序。由于这些潜在的信号处理能力,同源寡聚化在信号转导中发挥的作用比以前想象的要广泛得多。











































 京公网安备 11010802027423号
京公网安备 11010802027423号