Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coiled-coil registry shifts in the F684I mutant of Bicaudal D result in cargo-independent activation of dynein motility.
Traffic ( IF 3.6 ) Pub Date : 2020-05-07 , DOI: 10.1111/tra.12734 Heying Cui 1 , M Yusuf Ali 2 , Puja Goyal 1 , Kaiqi Zhang 1 , Jia Ying Loh 1 , Kathleen M Trybus 2 , Sozanne R Solmaz 1
Traffic ( IF 3.6 ) Pub Date : 2020-05-07 , DOI: 10.1111/tra.12734 Heying Cui 1 , M Yusuf Ali 2 , Puja Goyal 1 , Kaiqi Zhang 1 , Jia Ying Loh 1 , Kathleen M Trybus 2 , Sozanne R Solmaz 1
Affiliation
|
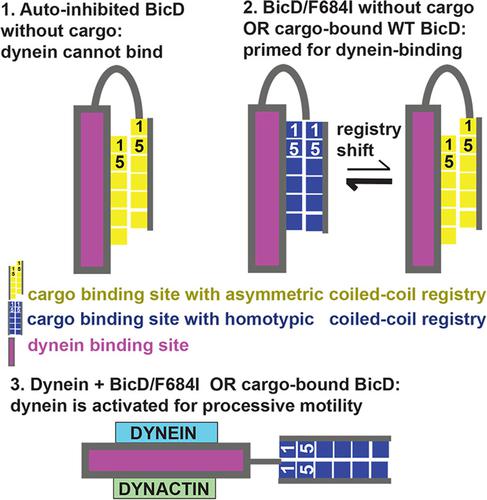
The dynein adaptor Drosophila Bicaudal D (BicD) is auto‐inhibited and activates dynein motility only after cargo is bound, but the underlying mechanism is elusive. In contrast, we show that the full‐length BicD/F684I mutant activates dynein processivity even in the absence of cargo. Our X‐ray structure of the C‐terminal domain of the BicD/F684I mutant reveals a coiled‐coil registry shift; in the N‐terminal region, the two helices of the homodimer are aligned, whereas they are vertically shifted in the wild‐type. One chain is partially disordered and this structural flexibility is confirmed by computations, which reveal that the mutant transitions back and forth between the two registries. We propose that a coiled‐coil registry shift upon cargo‐binding activates BicD for dynein recruitment. Moreover, the human homolog BicD2/F743I exhibits diminished binding of cargo adaptor Nup358, implying that a coiled‐coil registry shift may be a mechanism to modulate cargo selection for BicD2‐dependent transport pathways.
中文翻译:

Bicaudal D的F684I突变体中的螺旋线圈注册表移位导致独立于货物的动力蛋白活化。
动力蛋白适配器果蝇Bicaudal D(BicD)是自动抑制的,只有在货物绑定后才能激活动力蛋白,但其潜在机制尚不清楚。相比之下,我们表明,即使在没有货物的情况下,全长BicD / F684I突变体仍可激活动力蛋白的合成能力。我们的BicD / F684I突变体C末端结构域的X射线结构揭示了螺旋线圈注册表移位。在N末端区域,同型二聚体的两个螺旋排列成一条直线,而在野生型中则垂直移动。一条链是部分无序的,这种结构的灵活性已通过计算得到证实,这表明该突变体在两个注册表之间来回转换。我们建议,在装订货物后进行盘绕卷登记,以激活BicD进行动力蛋白的招募。此外,
更新日期:2020-06-29
中文翻译:

Bicaudal D的F684I突变体中的螺旋线圈注册表移位导致独立于货物的动力蛋白活化。
动力蛋白适配器果蝇Bicaudal D(BicD)是自动抑制的,只有在货物绑定后才能激活动力蛋白,但其潜在机制尚不清楚。相比之下,我们表明,即使在没有货物的情况下,全长BicD / F684I突变体仍可激活动力蛋白的合成能力。我们的BicD / F684I突变体C末端结构域的X射线结构揭示了螺旋线圈注册表移位。在N末端区域,同型二聚体的两个螺旋排列成一条直线,而在野生型中则垂直移动。一条链是部分无序的,这种结构的灵活性已通过计算得到证实,这表明该突变体在两个注册表之间来回转换。我们建议,在装订货物后进行盘绕卷登记,以激活BicD进行动力蛋白的招募。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号