当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Radiosynthesis and Biological Evaluation of Buprenorphine-Derived Phenylazocarboxamides as Novel μ-Opioid Receptor Ligands.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-06 , DOI: 10.1002/cmdc.202000180 Jasmin Krüll 1 , Stefanie K Fehler 1 , Laura Hofmann 1 , Natascha Nebel 2 , Simone Maschauer 2 , Olaf Prante 2 , Peter Gmeiner 1 , Harald Lanig 3 , Harald Hübner 1 , Markus R Heinrich 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-05-06 , DOI: 10.1002/cmdc.202000180 Jasmin Krüll 1 , Stefanie K Fehler 1 , Laura Hofmann 1 , Natascha Nebel 2 , Simone Maschauer 2 , Olaf Prante 2 , Peter Gmeiner 1 , Harald Lanig 3 , Harald Hübner 1 , Markus R Heinrich 1
Affiliation
|
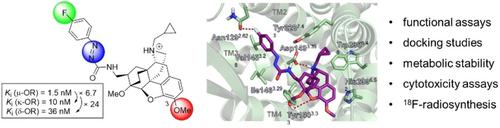
Targeted structural modifications have led to a novel type of buprenorphine‐derived opioid receptor ligand displaying an improved selectivity profile for the μ‐OR subtype. On this basis, it is shown that phenylazocarboxamides may serve as useful bioisosteric replacements for the widely occurring cinnamide units, without loss of OR binding affinity or subtype selectivity. This study further includes functional experiments pointing to weak partial agonist properties of the novel μ‐OR ligands, as well as docking and metabolism experiments. Finally, the unique bifunctional character of phenylazocarboxylates, herein serving as precursors for the azocarboxamide subunit, was exploited to demonstrate the accessibility of an 18F‐fluorinated analogue.
中文翻译:

作为新型μ-阿片受体配体的丁丙诺啡衍生的苯基偶氮甲酰胺的合成、放射合成和生物学评价。
有针对性的结构修饰导致了一种新型的丁丙诺啡衍生的阿片受体配体,对 μ-OR 亚型表现出改进的选择性。在此基础上,结果表明,苯基偶氮甲酰胺可以作为广泛存在的肉桂酰胺单元的有用的生物等排替代品,而不损失OR结合亲和力或亚型选择性。这项研究还包括指出新型 μ-OR 配体较弱的部分激动剂特性的功能实验,以及对接和代谢实验。最后,利用苯偶氮甲酸酯的独特双功能特征(本文用作偶氮甲酰胺亚基的前体)来证明18 F-氟化类似物的可及性。
更新日期:2020-07-03
中文翻译:

作为新型μ-阿片受体配体的丁丙诺啡衍生的苯基偶氮甲酰胺的合成、放射合成和生物学评价。
有针对性的结构修饰导致了一种新型的丁丙诺啡衍生的阿片受体配体,对 μ-OR 亚型表现出改进的选择性。在此基础上,结果表明,苯基偶氮甲酰胺可以作为广泛存在的肉桂酰胺单元的有用的生物等排替代品,而不损失OR结合亲和力或亚型选择性。这项研究还包括指出新型 μ-OR 配体较弱的部分激动剂特性的功能实验,以及对接和代谢实验。最后,利用苯偶氮甲酸酯的独特双功能特征(本文用作偶氮甲酰胺亚基的前体)来证明18 F-氟化类似物的可及性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号