当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, antiproliferative, cell cytotoxicity activity, DNA binding features and molecular d ocking study of novel enamine derivatives
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-06-25 , DOI: 10.1002/cbdv.202000139 Meliha Burcu Gürdere 1 , Ali Aydin 2 , Belkız Yencilek 1 , Fatih Ertürk 3 , Oğuz Özbek 1 , Sultan Erkan 4 , Yakup Budak 1 , Mustafa Ceylan 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-06-25 , DOI: 10.1002/cbdv.202000139 Meliha Burcu Gürdere 1 , Ali Aydin 2 , Belkız Yencilek 1 , Fatih Ertürk 3 , Oğuz Özbek 1 , Sultan Erkan 4 , Yakup Budak 1 , Mustafa Ceylan 1
Affiliation
|
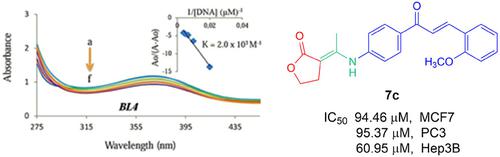
Novel enamine derivatives were synthesized from the reaction of lactone and chalcones and their antiproliferative and cytotoxic activities against six cancer cell lines (e. g., HeLa, HT29, A549, MCF7, PC3 and Hep3B) and one normal cell lines (e. g., FL) were investigated along with their mode of interactions with CT‐DNA. Most of the enamine derivatives with IC50 values of 86–168 μM demonstrated much stronger antiproliferative activity than the starting molecules against the cancer cells. While, among the enamine derivatives, four compounds displayed higher cytotoxic potency than the control drugs (5‐fluorouracil and cisplatin) against the Hep3B cell lines, these compounds did not exhibit any significant toxicity against normal cells, FL. The UV/VIS spectral data suggest that eight compounds cause hypochromism with a slight bathochromic shift (∼6 nm), indicating that they bind to the DNA by way of an intercalative or minor groove binding mode. The binding constants of the compounds are in the range of 0.1×103 M−1–2.3×104 M−1. The antiproliferative activity of studied enamine derivatives could possibly be due to their DNA binding as well as their cytotoxic properties. In addition to these assays, the chalcones and enamine derivatives were investigated by molecular docking to calculate the synergistic effect of antiproliferative activities against six human cancer cell lines.
中文翻译:

新型烯胺衍生物的合成、抗增殖、细胞毒活性、DNA结合特征和分子对接研究
通过内酯和查耳酮的反应合成了新的烯胺衍生物,并研究了它们对六种癌细胞系(例如 HeLa、HT29、A549、MCF7、PC3 和 Hep3B)和一种正常细胞系(例如,FL)的抗增殖和细胞毒活性以及它们与 CT-DNA 的相互作用模式。大多数 IC50 值为 86-168 μM 的烯胺衍生物表现出比起始分子更强的抗癌细胞增殖活性。虽然在烯胺衍生物中,四种化合物对 Hep3B 细胞系显示出比对照药物(5-氟尿嘧啶和顺铂)更高的细胞毒性效力,但这些化合物对正常细胞 FL 没有表现出任何显着的毒性。UV/VIS 光谱数据表明,八种化合物会导致具有轻微红移(~6 nm)的低色度,表明它们通过嵌入或小沟结合模式与 DNA 结合。化合物的结合常数在0.1×103 M-1–2.3×104 M-1范围内。所研究的烯胺衍生物的抗增殖活性可能是由于它们的 DNA 结合以及它们的细胞毒特性。除了这些测定之外,还通过分子对接研究了查耳酮和烯胺衍生物,以计算抗增殖活性对六种人类癌细胞系的协同作用。所研究的烯胺衍生物的抗增殖活性可能是由于它们的 DNA 结合以及它们的细胞毒特性。除了这些测定之外,还通过分子对接研究了查耳酮和烯胺衍生物,以计算抗增殖活性对六种人类癌细胞系的协同作用。所研究的烯胺衍生物的抗增殖活性可能是由于它们的 DNA 结合以及它们的细胞毒特性。除了这些测定之外,还通过分子对接研究了查耳酮和烯胺衍生物,以计算抗增殖活性对六种人类癌细胞系的协同作用。
更新日期:2020-06-25
中文翻译:

新型烯胺衍生物的合成、抗增殖、细胞毒活性、DNA结合特征和分子对接研究
通过内酯和查耳酮的反应合成了新的烯胺衍生物,并研究了它们对六种癌细胞系(例如 HeLa、HT29、A549、MCF7、PC3 和 Hep3B)和一种正常细胞系(例如,FL)的抗增殖和细胞毒活性以及它们与 CT-DNA 的相互作用模式。大多数 IC50 值为 86-168 μM 的烯胺衍生物表现出比起始分子更强的抗癌细胞增殖活性。虽然在烯胺衍生物中,四种化合物对 Hep3B 细胞系显示出比对照药物(5-氟尿嘧啶和顺铂)更高的细胞毒性效力,但这些化合物对正常细胞 FL 没有表现出任何显着的毒性。UV/VIS 光谱数据表明,八种化合物会导致具有轻微红移(~6 nm)的低色度,表明它们通过嵌入或小沟结合模式与 DNA 结合。化合物的结合常数在0.1×103 M-1–2.3×104 M-1范围内。所研究的烯胺衍生物的抗增殖活性可能是由于它们的 DNA 结合以及它们的细胞毒特性。除了这些测定之外,还通过分子对接研究了查耳酮和烯胺衍生物,以计算抗增殖活性对六种人类癌细胞系的协同作用。所研究的烯胺衍生物的抗增殖活性可能是由于它们的 DNA 结合以及它们的细胞毒特性。除了这些测定之外,还通过分子对接研究了查耳酮和烯胺衍生物,以计算抗增殖活性对六种人类癌细胞系的协同作用。所研究的烯胺衍生物的抗增殖活性可能是由于它们的 DNA 结合以及它们的细胞毒特性。除了这些测定之外,还通过分子对接研究了查耳酮和烯胺衍生物,以计算抗增殖活性对六种人类癌细胞系的协同作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号