当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Jet-Lagged Nanoparticles Enhanced Immunotherapy Efficiency through Synergistic Reconstruction of Tumor Microenvironment and Normalized Tumor Vasculature.
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2020-05-06 , DOI: 10.1002/adhm.202000075 Zhijie Jiang 1 , Hui Xiong 1 , Shan Yang 1 , Yun Lu 2 , Yudi Deng 1 , Jianxu Yao 1 , Jing Yao 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2020-05-06 , DOI: 10.1002/adhm.202000075 Zhijie Jiang 1 , Hui Xiong 1 , Shan Yang 1 , Yun Lu 2 , Yudi Deng 1 , Jianxu Yao 1 , Jing Yao 1
Affiliation
|
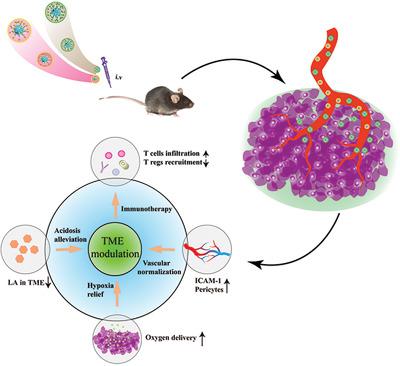
Lactic acid (LA), an anaerobic glycolysis metabolite normally oversecreted by tumor cells, can inhibit the activity of T cells and stimulate the rapid proliferation and migration of tumor endothelial cells (TECs), thereby limiting the synergistic treatment efficiency of tumor immunotherapy and vascular normalization. Herein, Jet‐lagged nanoparticles, apatinib (APA)‐loaded TEC‐targeting nanodrug (APA/MCP) and lonidamine (LND)‐loaded tumor cell‐targeting nanodrug (LND/MCA), are constructed to combine vascular normalization therapy and tumor cell metabolic treatment. APA/MCP can block VEGF/VEGFR2 to inhibit TEC proliferation and LND/MCA can inhibit LA efflux to remodel tumor acid metabolism. After treatment, Jet‐lagged nanoparticles remarkably reduce the level of LA in tumor microenvironment (TME) through limiting LA efflux. Besides, the pericyte cell coverage ratio of tumor vasculature increased to 69%, which is significantly improved compared to the APA/MCP group (47%). Moreover, the results of in vivo pharmacodynamic studies show that after the above synergistic reconstruction of TME and normalized tumor vasculature, the therapeutic effect of programmed death 1 (PD‐1) drug is improved 3‐folds to that of the PD‐1 group. Above all, the strategy in this paper may propose an innovative vision to facilitate the tumor immunotherapy through high‐precision spatiotemporal delivery strategy of nanodrugs.
中文翻译:

通过协同重建肿瘤微环境和规范化的肿瘤脉管,Jet滞后的纳米颗粒增强了免疫治疗效率。
乳酸(LA)是一种通常被肿瘤细胞过度分泌的厌氧糖酵解代谢产物,可抑制T细胞的活性并刺激肿瘤内皮细胞(TECs)的快速增殖和迁移,从而限制了肿瘤免疫疗法和血管正常化的协同治疗效率。在此,构建了喷射滞后纳米颗粒,载有阿帕替尼(APA)的TEC靶向纳米药物(APA / MCP)和载有LONID胺(LND)的肿瘤细胞靶向纳米药物(LND / MCA),以结合血管正常化治疗和肿瘤细胞代谢治疗。APA / MCP可以阻断VEGF / VEGFR2抑制TEC的增殖,而LND / MCA则可以抑制LA外排以重塑肿瘤的酸代谢。治疗后,Jet滞后的纳米颗粒通过限制LA外排显着降低了肿瘤微环境(TME)中的LA水平。除了,肿瘤血管的周细胞覆盖率增加到69%,与APA / MCP组(47%)相比有显着提高。此外,体内药效学研究的结果表明,经过上述TME和正常肿瘤血管的协同重建,程序性死亡1(PD-1)药物的治疗效果比PD-1组提高了3倍。最重要的是,本文中的策略可能提出了一种创新的愿景,即通过纳米药物的高精度时空传递策略来促进肿瘤免疫治疗。程序性死亡1(PD-1)药物的治疗效果比PD-1组提高了3倍。最重要的是,本文中的策略可能提出了一种创新的愿景,即通过纳米药物的高精度时空传递策略来促进肿瘤免疫治疗。程序性死亡1(PD-1)药物的治疗效果比PD-1组提高了3倍。最重要的是,本文中的策略可能提出了一种创新的愿景,即通过纳米药物的高精度时空传递策略来促进肿瘤免疫治疗。
更新日期:2020-06-24
中文翻译:

通过协同重建肿瘤微环境和规范化的肿瘤脉管,Jet滞后的纳米颗粒增强了免疫治疗效率。
乳酸(LA)是一种通常被肿瘤细胞过度分泌的厌氧糖酵解代谢产物,可抑制T细胞的活性并刺激肿瘤内皮细胞(TECs)的快速增殖和迁移,从而限制了肿瘤免疫疗法和血管正常化的协同治疗效率。在此,构建了喷射滞后纳米颗粒,载有阿帕替尼(APA)的TEC靶向纳米药物(APA / MCP)和载有LONID胺(LND)的肿瘤细胞靶向纳米药物(LND / MCA),以结合血管正常化治疗和肿瘤细胞代谢治疗。APA / MCP可以阻断VEGF / VEGFR2抑制TEC的增殖,而LND / MCA则可以抑制LA外排以重塑肿瘤的酸代谢。治疗后,Jet滞后的纳米颗粒通过限制LA外排显着降低了肿瘤微环境(TME)中的LA水平。除了,肿瘤血管的周细胞覆盖率增加到69%,与APA / MCP组(47%)相比有显着提高。此外,体内药效学研究的结果表明,经过上述TME和正常肿瘤血管的协同重建,程序性死亡1(PD-1)药物的治疗效果比PD-1组提高了3倍。最重要的是,本文中的策略可能提出了一种创新的愿景,即通过纳米药物的高精度时空传递策略来促进肿瘤免疫治疗。程序性死亡1(PD-1)药物的治疗效果比PD-1组提高了3倍。最重要的是,本文中的策略可能提出了一种创新的愿景,即通过纳米药物的高精度时空传递策略来促进肿瘤免疫治疗。程序性死亡1(PD-1)药物的治疗效果比PD-1组提高了3倍。最重要的是,本文中的策略可能提出了一种创新的愿景,即通过纳米药物的高精度时空传递策略来促进肿瘤免疫治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号