Free Radical Biology and Medicine ( IF 7.4 ) Pub Date : 2020-05-07 , DOI: 10.1016/j.freeradbiomed.2020.04.024 Matthew J Smith 1 , Mark Fowler 2 , Richard J Naftalin 1 , Richard C M Siow 1
|
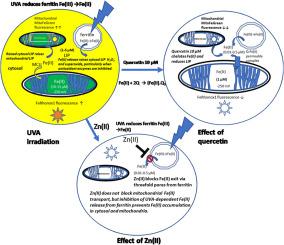
UVA irradiation of human dermal fibroblasts and endothelial cells induces an immediate transient increase in cytosolic Fe(II), as monitored by the fluorescence Fe(II) reporters, FeRhonox1 in cytosol and MitoFerroGreen in mitochondria. Both superoxide dismutase (SOD) inhibition by tetrathiomolybdate (ATM) and catalase inhibition by 3-amino-1, 2, 4-triazole (ATZ) increase and prolong the cytosolic Fe(II) signal after UVA irradiation. SOD inhibition with ATM also increases mitochondrial Fe(II). Thus, mitochondria do not source the UV-dependent increase in cytosolic Fe(II), but instead reflect and amplify raised cytosolic labile Fe(II) concentration. Hence control of cytosolic ferritin iron release is key to preventing UVA-induced inflammation. UVA irradiation also increases dermal endothelial cell H2O2, as monitored by the adenovirus vector Hyper-DAAO-NES(HyPer). These UVA-dependent changes in intracellular Fe(II) and H2O2 are mirrored by increases in cell superoxide, monitored with the luminescence probe L-012. UV-dependent increases in cytosolic Fe(II), H2O2 and L-012 chemiluminescence are prevented by ZnCl2 (10 μM), an effective inhibitor of Fe(II) transport via ferritin's 3-fold channels. Quercetin (10 μM), a potent membrane permeable Fe(II) chelator, abolishes the cytosolic UVA-dependent FeRhonox1, Fe(II) and HyPer, H2O2 and increase in MitoFerroGreen Fe(II) signals. The time course of the quercetin-dependent decrease in endothelial H2O2 correlates with the decrease in FeRhox1 signal and both signals are fully suppressed by preloading cells with ZnCl2. These results confirm that antioxidant enzyme activity is the key factor in controlling intracellular iron levels, and hence maintenance of cell antioxidant capacity is vitally important in prevention of inflammation initiated by labile iron and UVA.
中文翻译:

UVA辐射增加了人类皮肤成纤维细胞和内皮细胞铁蛋白中亚铁的释放:细胞衰老和衰老的后果。
UVA照射人皮肤成纤维细胞和内皮细胞可引起胞质Fe(II)的瞬时瞬时增加,如荧光Fe(II)报道分子,细胞溶质中的FeRhonox1和线粒体中的MitoFerroGreen所监测。四硫代钼酸盐(ATM)对超氧化物歧化酶(SOD)的抑制和3-氨基-1、2、4-三唑(ATZ)对过氧化氢酶的抑制都增加并延长了UVA照射后的胞质Fe(II)信号。用ATM抑制SOD也会增加线粒体Fe(II)。因此,线粒体不引起胞质Fe(II)的紫外线依赖性增加,而是反映并放大了胞质不稳定Fe(II)浓度的升高。因此,控制胞质铁蛋白铁释放是预防UVA引起的炎症的关键。UVA照射还会增加真皮内皮细胞H 2 O如图2所示,由腺病毒载体Hyper-DAAO-NES(HyPer)监测。这些细胞内Fe(II)和H 2 O 2中UVA依赖性的变化通过细胞超氧化物的增加反映出来,用发光探针L-012进行监测。ZnCl 2(10μM)阻止了UV依赖性的胞质Fe(II),H 2 O 2和L-012化学发光的增加,ZnCl 2是有效的Fe(II)通过铁蛋白3倍通道运输的抑制剂。槲皮素(10μM)是有效的膜可渗透性Fe(II)螯合剂,可消除依赖于UVA的胞质FeRhonox1,Fe(II)和HyPer,H 2 O 2,并增加MitoFerroGreen Fe(II)信号。槲皮素依赖性减少内皮细胞H的时间过程2 O 2与FeRhox1信号的减少有关,并且通过用ZnCl 2预加载细胞完全抑制了这两个信号。这些结果证实,抗氧化酶活性是控制细胞内铁水平的关键因素,因此维持细胞抗氧化能力对于预防不稳定铁和UVA引发的炎症至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号