当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thinking Outside the Cage: A New Hypothesis That Accounts for Variable Yields of Radicals from the Reaction of CO2 with ONOO.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-05-07 , DOI: 10.1021/acs.chemrestox.9b00309 Willem H Koppenol 1 , Sandra Serrano-Luginbuehl 2 , Thomas Nauser 3 , Reinhard Kissner 3
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-05-07 , DOI: 10.1021/acs.chemrestox.9b00309 Willem H Koppenol 1 , Sandra Serrano-Luginbuehl 2 , Thomas Nauser 3 , Reinhard Kissner 3
Affiliation
|
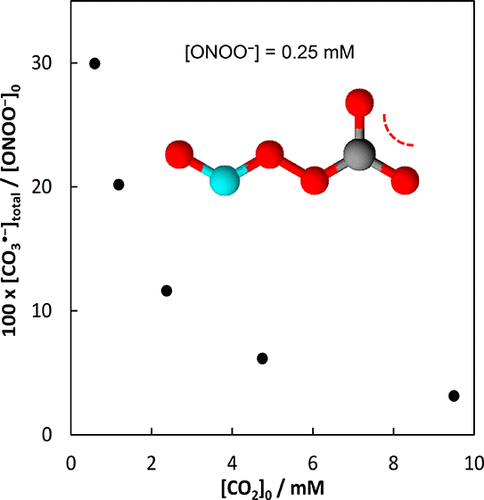
In biology, the reaction of ONOO– with CO2 is the main sink for ONOO–. This reaction yields CO3•–, NO2•, NO3–, and CO2. There is a long-standing debate with respect to the yield of the radicals relative to ONOO–. The reaction of ONOO– with CO2 results at first in ONOOCO2–. According to one hypothesis, ONOOCO2– is extremely short-lived and devolves into a solvent cage that contains CO3•– and NO2•. Of these solvent cages, approximately two/thirds result in NO3– and CO2, and approximately one/third release CO3•– and NO2• that oxidize the substrate. According to our hypothesis, ONOOCO2– is formed much faster, is relatively long-lived, and may also be an oxidant; the limited yield is the result of ONOOCO2– being scavenged by a second CO2 under conditions of a high CO2 concentration. We disagree with the first hypothesis for three reasons: First, it is based on an estimated K for the reaction of ONOO– with CO2 to form ONOOCO2– of ∼1 M–1, while experiments yield a value of 4.5 × 103 M–1. Second, we argue that the solvent cage as proposed is physically not realistic. Given the less than diffusion-controlled rate constant of CO3•– with NO2•, all radicals would escape from the solvent cage. Third, the reported ∼33% radical is not supported by an experiment where mass balance was established. We propose here a hybrid mechanism. After formation of ONOOCO2–, it undergoes homolysis to yield CO3•– with NO2•, or, depending on [CO2], it is scavenged by a second CO2; CO3•– oxidizes ONOO–, if present. These reactions allow us to successfully simulate the reaction of ONOO– with CO2 over a wide range of ONOO–/CO2 ratios. At lower ratios, fewer radicals are formed, while at higher ratios, radical yields between 30% and 40% are predicted. The differences in radical yields reported may thus be traced to the experimental ONOO–/CO2 ratios. Given a physiological [CO2] of 1.3 mM, the yield of CO3•– and NO2• is 19%, and lower if ONOOCO2– has a significant reactivity of its own.
中文翻译:

在笼子外思考:一种新的假说,该假说解释了CO2与ONOO反应产生的自由基的可变产率。
在生物学中,ONOO的反应-与CO 2是用于ONOO主水槽- 。该反应产生CO 3 •–,NO 2 •,NO 3 –和CO 2。有相对于相对于ONOO自由基的产量争论由来已久- 。ONOO –与CO 2的反应首先在ONOOCO 2 –中产生。根据一个假设,ONOOCO 2 -是极其短暂的和转予到含有CO溶剂笼3 • -和NO 2 •。这些溶剂笼,约两/三分之二导致NO 3 -和CO 2,和大约一/第三释放CO 3 • -和NO 2 •其氧化基材。根据我们的假设,ONOOCO 2 -快得多形成,相对长住,也可能是一种氧化剂; 产量有限的原因是ONOOCO 2 –在高CO 2浓度的条件下被第二种CO 2清除。我们不同意第一个假设,原因有以下三个:第一,它基于ONOO –与CO反应的估计K2形成约1 M –1的ONOOCO 2 –,而实验得出的值为4.5×10 3 M –1。其次,我们认为所提出的溶剂笼在物理上是不现实的。给定CO 3 •–小于NO 2 •的扩散控制速率常数,所有自由基都会从溶剂笼中逸出。第三,报告的〜33%的自由基不受建立质量平衡的实验的支持。我们在这里提出一种混合机制。形成ONOOCO 2 –后,将其进行均质分解以生成带有NO 2 •的CO 3 •–,或者取决于[CO2 ],它被第二个CO 2清除;CO 3 •–氧化ONOO –(如果存在)。这些反应使我们能够成功模拟ONOO –与CO 2在广泛的ONOO – / CO 2比率范围内的反应。在较低的比率下,形成的自由基较少,而在较高的比率下,预测的自由基产率为30%至40%。在报道基团的产率的差异因此可以追溯到实验ONOO - / CO 2比。假设生理[CO 2 ]为1.3 mM,则CO 3 •和NO 2 •的产率是19%,并降低如果ONOOCO 2 -拥有自己的显著反应。
更新日期:2020-05-07
中文翻译:

在笼子外思考:一种新的假说,该假说解释了CO2与ONOO反应产生的自由基的可变产率。
在生物学中,ONOO的反应-与CO 2是用于ONOO主水槽- 。该反应产生CO 3 •–,NO 2 •,NO 3 –和CO 2。有相对于相对于ONOO自由基的产量争论由来已久- 。ONOO –与CO 2的反应首先在ONOOCO 2 –中产生。根据一个假设,ONOOCO 2 -是极其短暂的和转予到含有CO溶剂笼3 • -和NO 2 •。这些溶剂笼,约两/三分之二导致NO 3 -和CO 2,和大约一/第三释放CO 3 • -和NO 2 •其氧化基材。根据我们的假设,ONOOCO 2 -快得多形成,相对长住,也可能是一种氧化剂; 产量有限的原因是ONOOCO 2 –在高CO 2浓度的条件下被第二种CO 2清除。我们不同意第一个假设,原因有以下三个:第一,它基于ONOO –与CO反应的估计K2形成约1 M –1的ONOOCO 2 –,而实验得出的值为4.5×10 3 M –1。其次,我们认为所提出的溶剂笼在物理上是不现实的。给定CO 3 •–小于NO 2 •的扩散控制速率常数,所有自由基都会从溶剂笼中逸出。第三,报告的〜33%的自由基不受建立质量平衡的实验的支持。我们在这里提出一种混合机制。形成ONOOCO 2 –后,将其进行均质分解以生成带有NO 2 •的CO 3 •–,或者取决于[CO2 ],它被第二个CO 2清除;CO 3 •–氧化ONOO –(如果存在)。这些反应使我们能够成功模拟ONOO –与CO 2在广泛的ONOO – / CO 2比率范围内的反应。在较低的比率下,形成的自由基较少,而在较高的比率下,预测的自由基产率为30%至40%。在报道基团的产率的差异因此可以追溯到实验ONOO - / CO 2比。假设生理[CO 2 ]为1.3 mM,则CO 3 •和NO 2 •的产率是19%,并降低如果ONOOCO 2 -拥有自己的显著反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号