当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Characterization of a Leucine-Based Block Co-Polypeptide: The Effect of the Leucine Zipper on Self-Assembly.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-05-07 , DOI: 10.1021/acs.biomac.0c00420 Brooke E Barnes 1 , Taylor A Jenkins 1 , Lauren M Stein 1 , Robert T Mathers 2 , Masita Wicaksana 3 , Melissa A Pasquinelli 4 , Daniel A Savin 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-05-07 , DOI: 10.1021/acs.biomac.0c00420 Brooke E Barnes 1 , Taylor A Jenkins 1 , Lauren M Stein 1 , Robert T Mathers 2 , Masita Wicaksana 3 , Melissa A Pasquinelli 4 , Daniel A Savin 1
Affiliation
|
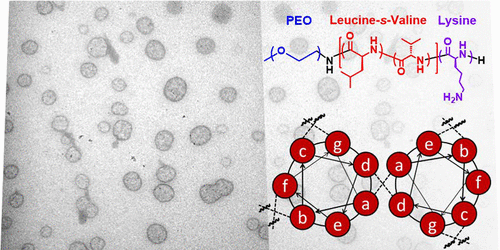
The self-assembly behavior of an ABC triblock copolypeptide consisting of poly(ethylene oxide-b-(leucine-s-valine)-b-lysine) (PEO−PLV−PK) was examined via dynamic light scattering in dilute aqueous solution. Leucine is a hydrophobic, α-helix forming polypeptide that exhibits a “zipper effect” in coiled-coil dimers. We hypothesize that the specific interaction afforded by the leucine zipper dominates the thermodynamics of self-assembly through the side-by-side ordering of α-helices, which drives vesicle formation in a polymer with only 6 wt % hydrophobic content. Additionally, a multitude of assembly sizes and morphologies were attainable from a single polymer, depending on the solution processing method. Thermodynamic effects of the leucine zipper can be interpreted, in part, from solubility parameters determined from molecular modeling. The combination of synthesis, solvent processing, and computational studies helps to elucidate the thermodynamic effects of this unique assembly motif on classical self-assembly processes.
中文翻译:

基于亮氨酸的嵌段共多肽的合成和表征:亮氨酸拉链对自组装的影响。
的ABC三嵌段共聚由聚(环氧乙烷-环氧的自组装行为b - (亮氨酸小号-缬氨酸) - b-赖氨酸(PEO-PLV-PK)通过在稀水溶液中的动态光散射进行检测。亮氨酸是疏水的,形成α-螺旋的多肽,在卷曲螺旋二聚体中表现出“拉链效应”。我们假设亮氨酸拉链提供的特异性相互作用通过α螺旋的并排排列控制了自组装的热力学,这驱动了只有6 wt%疏水含量的聚合物中的囊泡形成。另外,取决于溶液加工方法,可以由单一聚合物获得多种组装尺寸和形态。亮氨酸拉链的热力学作用可以部分地由分子模型确定的溶解度参数来解释。合成,溶剂处理,
更新日期:2020-05-07
中文翻译:

基于亮氨酸的嵌段共多肽的合成和表征:亮氨酸拉链对自组装的影响。
的ABC三嵌段共聚由聚(环氧乙烷-环氧的自组装行为b - (亮氨酸小号-缬氨酸) - b-赖氨酸(PEO-PLV-PK)通过在稀水溶液中的动态光散射进行检测。亮氨酸是疏水的,形成α-螺旋的多肽,在卷曲螺旋二聚体中表现出“拉链效应”。我们假设亮氨酸拉链提供的特异性相互作用通过α螺旋的并排排列控制了自组装的热力学,这驱动了只有6 wt%疏水含量的聚合物中的囊泡形成。另外,取决于溶液加工方法,可以由单一聚合物获得多种组装尺寸和形态。亮氨酸拉链的热力学作用可以部分地由分子模型确定的溶解度参数来解释。合成,溶剂处理,











































 京公网安备 11010802027423号
京公网安备 11010802027423号